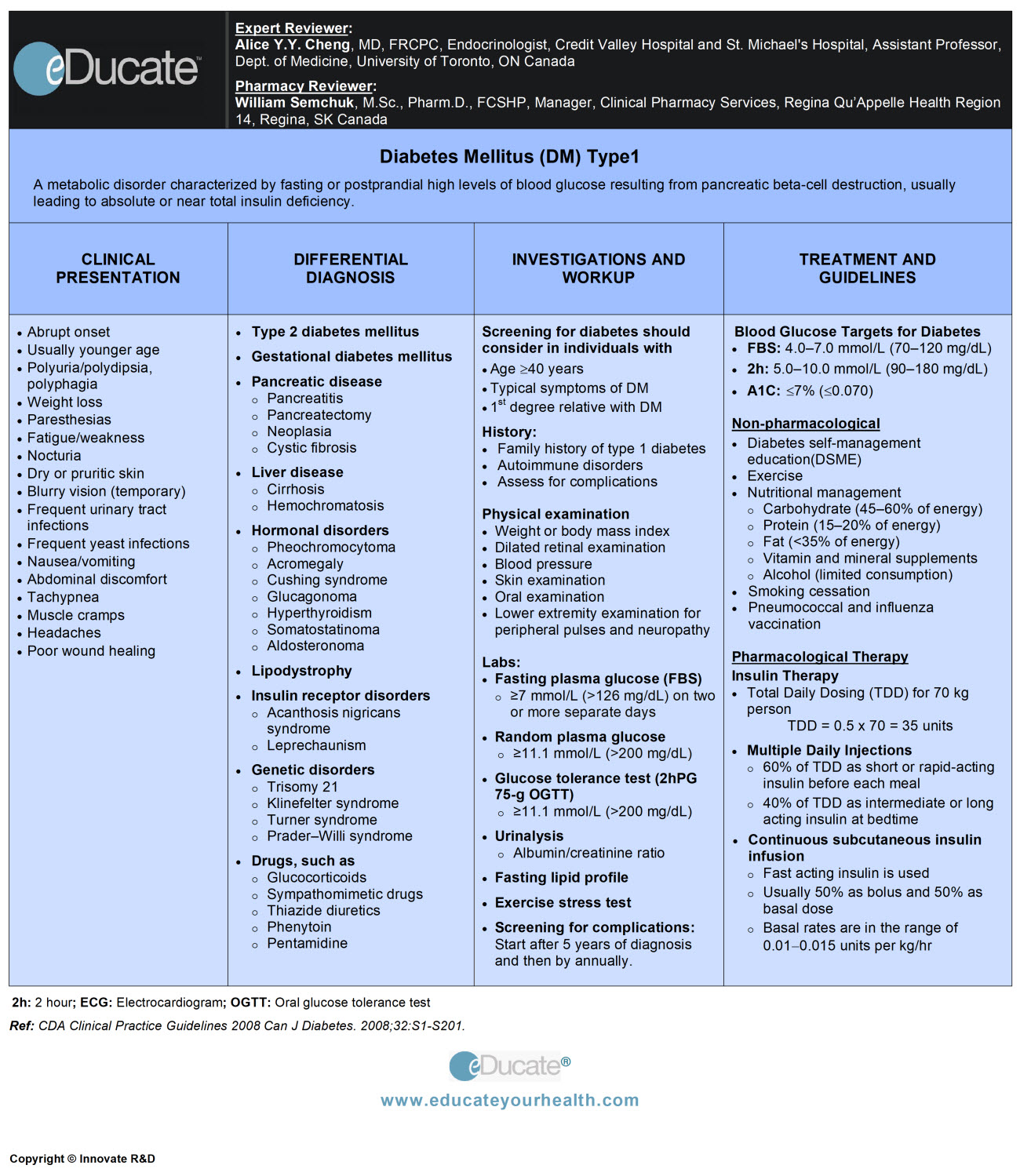
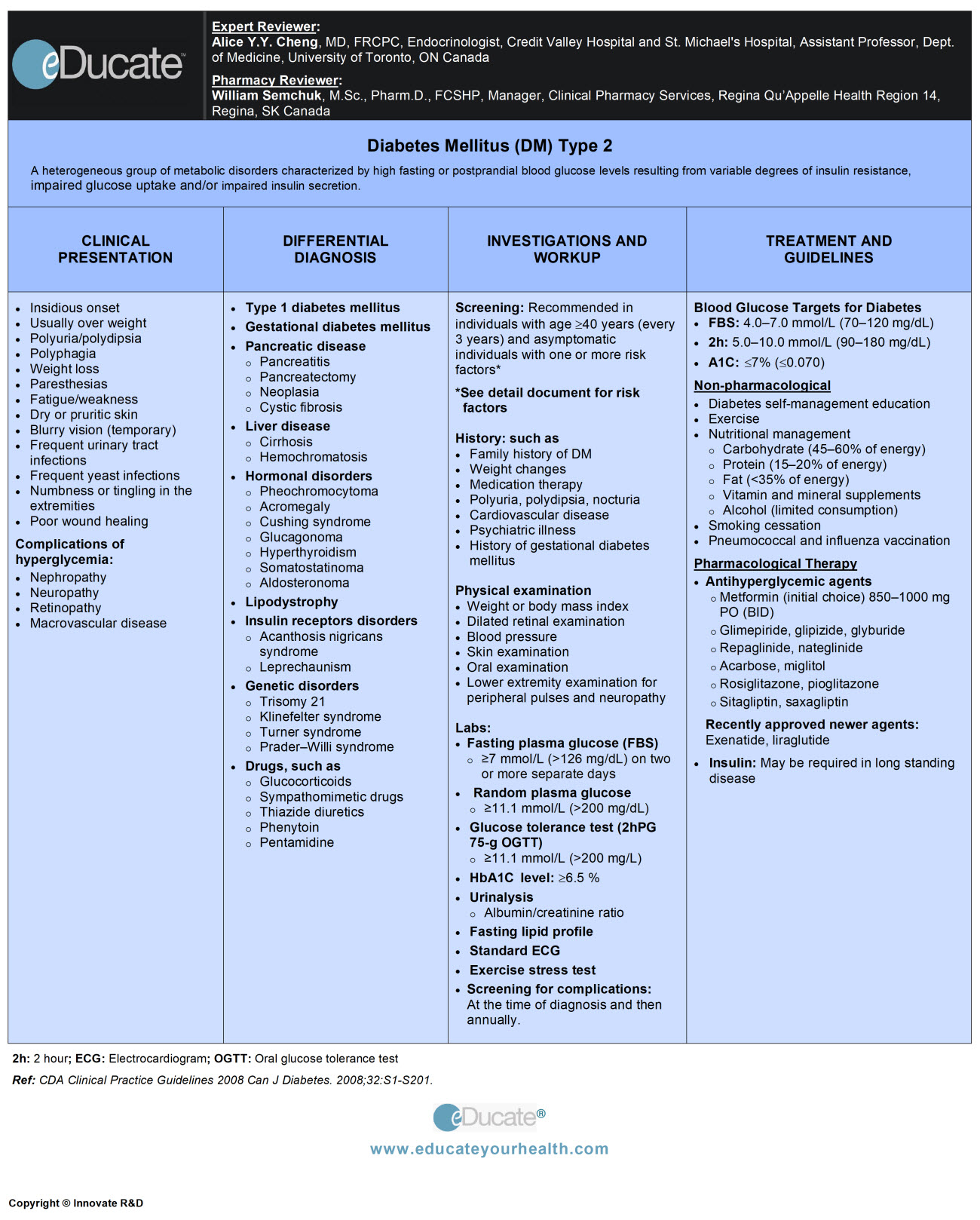
Diabetes Mellitus
EXPERT REVIEWER:
Alice Y.Y. Cheng, MD, FRCPC, Endocrinologist, Credit Valley Hospital and St. Michael’s Hospital, Assistant Professor, Dept. of Medicine, University of Toronto, ON Canada
PHARMACY REVIEWER:
William Semchuk, M.Sc., Pharm.D.,FCSHP Manager, Clinical Pharmacy Services, Regina Qu’Appelle Health Region 14, Regina, SK Canada
Definition
A condition of impaired carbohydrate metabolism characterized by fasting or postprandial high levels of blood glucose that results from inadequate insulin production, insulin resistance and/or impaired glucose uptake.
Prediabetes: Refers to blood glucose values intermediate between normal and overt diabetes; however, not everyone with prediabetes will develop type 2 diabetes.
Impaired fasting glucose: Fasting blood glucose 6.1-6.9 mmol/L [CDA – Clinical Practice Guidelines (CGP) 2008] or 100-125 mg/dL [American Association of Clinical Endocrinologists (AACE) DM Guidelines 2011]
Impaired glucose tolerance: Blood glucose measurement on a glucose tolerance test of 7.8-11.0 mmol/L in the 2-hour sample (CDA-CGP 2008) or 140-199 mg/dL (AACE Diabetes Mellitus Guidelines 2007)
CLASSIFICATION:
- Type1 Diabetes (T1DM): Results from the destruction of insulin-producing pancreatic beta cells leads to the absolute or near-total deficiency of insulin
- Type 2 Diabetes (T2DM): Characterized by a variable degree of insulin resistance and impaired secretion
- Gestational Diabetes (GDM): Any degree of glucose intolerance with onset or first recognition during pregnancy
- Other specific types: Uncommon conditions or diabetes associated with other diseases or drug use
Emergencies and conditions associated with diabetes mellitus
Etiology
Type 1 diabetes mellitus (T1DM): Formerly called insulin-dependent diabetes or juvenile-onset diabetes

Risk Factors
- Certain human leukocyte antigen (HLA) types, such as HLA DR3 and/or DR4 haplotype (often seen in people with type 1A DM)
- Presence of a specific 64,000 mw protein that may be responsible for antibody formation
- Family history of diabetes in 1st-degree relatives
- Approx. 30-70% concordance in identical twins
Type 2 diabetes mellitus (T2DM): Formerly called non-insulin-dependent diabetes or adult-onset diabetes
Insulin resistance and relative deficiency of insulin secretion are central causes of Type 2 DM.
- Genetic factors: >70% concordance in monozygotic twins
- Immune-mediated: Such as transplant recipients
- Drug- or chemical-induced: Such as, corticosteroids and some anti-psychotics
- Infection: e.g. HIV
- Systemic diseases: Hemochromatosis, Cushing’s disease, acromegaly
Risk Factors
- Age ≥40 years
- Family history of type 2 DM: 1st-degree relative
- Race/ethnicity:
- Aboriginal/Hispanic/Asian/African/Pacific Islander
- History of impaired fasting glucose (IFG) or impaired glucose tolerance (IGT)
- History of vascular disease
- History of gestational diabetes (GDM)
- History of delivery of a macrosomic infant: wt. >4 kg (>9 lbs)
- Hypertension (blood pressure >140/90 mmHg)
- Dyslipidemia
- Obesity/overweight (body mass index >25 kg/m2)
- Particularly visceral or central obesity
- Polycystic ovary syndrome
- Acanthosis nigricans
- Schizophrenia
Gestational diabetes
Risk factors
- Advancing maternal age
- Family history of DM in the first-degree relative
- Race/ethnicity:
- Aboriginal/Hispanic/Asian/African/Pacific Islander
- History of abnormal glucose metabolism
- Low fiber and high glycemic index diet
- Overweight or obese state
- Polycystic ovarian syndrome
- Prior gestational diabetes (GDM)
- Approximately 80% chances in subsequent pregnancies
- Sedentary lifestyle
Epidemiology
Prevalence:
- International Diabetes Foundation 2010 worldwide estimates: Approx. 285 million cases
- Canadian Diabetes Association (CDA) 2010: 7.6% of the Canadian population has diabetes that means 2.7 million Canadians are affected. It is estimated that this will rise to 4.2 million or 10.8% of the population by 2020
Factors influencing the prevalence and incidence of diabetes include:
- Age: Increases with aging
- Sex: Men > Women
- Race: First Nations (data from British Columbia suggests the prevalence to be 40% higher than other residents) – (www.ndss.gc.ca)
U.S. Centers for Disease Control and Prevention (CDC) 2005 estimates: Approx. 20.8 million persons, or 7% of the population
Race (age >20 years)
- African Americans: Approx. 13.6%
- Latinos: Approx. 10%
- Native Americans: Approx. 15%
Type 1 DM
- Mean age of onset: 8-12 years
- Sex: Onset 1.5 years earlier in girls than boys
- Incidence: 15/100,000 per year
Type 2 DM
Incidence
- Sex:
- Male: 230/100,000
- Female: 340/100,000
- Race:
- Whites 7.2%
- Hispanics 9%
- Blacks 11.2%
- Pima Indians 35%
- Age: Usually after age 40; however, but can develop at any age
- Weight: Higher chances among overweight children and adolescents
Gestational diabetes
Affects ~2-4% of pregnant Canadian women (~8-18% in some ethnic communities) and ~3-5% pregnant women in the US depending on their risk factors.
Increased risk for:
- Perinatal mortality and morbidity
- Development of diabetes later in life
Ref: Report from the National Diabetes Surveillance System: Diabetes in Canada, 2009.
Pathophysiology
Decreased insulin secretion by the pancreatic beta cells and/or decrease in insulin action leads to reduced intracellular uptake of glucose causing increased blood glucose levels i.e. hyperglycemia.
Type 1 DM
Autoimmune and/or idiopathic process effect on pancreatic beta cells causing decreased or no production of insulin resulting in increased blood glucose levels (hyperglycemia).
Type 2 DM
Mainly insulin resistance and relative insulin deficiency leads to decreased glucose transport in muscle, impaired hepatic glucose production, altered fat metabolism, resulting in metabolic dysfunction; increased blood glucose, lipid and protein levels.
Prolonged hyperglycemia leads to:
- Osmotic diuresis due to glucosuria
- Hypovolemia and hemoconcentration due to loss of water, sodium, and potassium
- Hyperosmolarity due to excessive blood sugar and increasing sodium concentration
- Tissue hypoxia due to decreased blood flow to the organs, because of increased blood viscosity
- Neurologic signs and symptoms due to intracellular fluid and electrolyte shift
Gestational diabetes
Metabolic changes of normal pregnancy occur in:
Early pregnancy
- Maternal estrogen and progesterone increase and promote pancreatic β-cell hyperplasia, resulting in increased insulin release
Later in pregnancy (2nd trimester through the 3rd)
Insulin resistance develops in peripheral tissues due to:
- Increased hormonal levels of human placental lactogen (HPL)
- Increased adipose deposition
- Decreased exercise
- Increased caloric intake
Gestational diabetes (GDM) develops when maternal insulin secretion is not sufficient to meet increased demand secondary to marked resistance.
Clinical Presentation
General features in all types:
- Polyuria/polydipsia/polyphagia
- Weight loss/fatigue/weakness
- Paraesthesias
- Blurry vision (resolves as hyperglycemia is controlled)
- Frequent urinary tract and yeast infections
- Dry or pruritic skin
- Poor wound healing
Type 1 diabetes mellitus (T1DM)
- Usually younger age
- Nausea/vomiting/abdominal discomfort
- Muscle cramps
- Headaches
Diabetic ketoacidosis (DKA)
- Usually develops rapidly, in less than 24 hours
- Hypotension/tachycardia
- Hypothermia or fever
- Tachypnea/fruity odor to breath (acetone smell)
- Kussmaul respirations (deep and labored breathing)
- Decreased reflexes
- Abdominal pain/ileus
- Dry mucous membrane/decreased perspiration
- Confusion/coma
Type 2 diabetes mellitus (T2DM)
General features of T2DM and/or hyperglycemic complications include nephropathy, neuropathy, retinopathy, and cardiovascular disease.
Individuals with the hyperosmolar hyperglycemic state (HHS) developing over days; may present as follows:
- Weight loss/dehydration
- Hypotension/tachycardia
- Poor skin turgor and dryness
- Lethargy/somnolence/confusion
Late presentation
- Hallucinations/aphasia/seizures
- Nystagmus/hemianopsia/hemiplegia
- Altered mental status/coma
Hypoglycemia:
Can occur in both type 1 and type 2 diabetes mellitus
- Hunger/sweating
- Palpitations/tachycardia
- Dizziness/light-headedness
- Nausea/headache
- Blurred vision
- Lethargy/drowsiness
- Confusion/anxiety/irritability
- Weakness/slurred speech
- Tingling/paresthesias
- Seizure/coma
Metabolic syndrome (association)
- Mainly results because of central abdominal obesity and insulin resistance
- Characterized by clustering of metabolic abnormalities, including obesity, abdominal obesity, elevated triglycerides, HDL cholesterol, hypertension, and impaired fasting plasma glucose/DM
- The diagnostic criteria are not universal and vary among governing organizations e.g. International Diabetes Federation (IDF), World Health Organization, and National Cholesterol Education Program (NCEP)
Chronic complications of diabetes mellitus
Microvascular:
A) Diabetic retinopathy
Macular edema (ME): Leads to decreased visual acuity and exhibits
- Retinal thickening and edema involving the macula
- Hard exudates
Nonproliferative diabetic retinopathy (NPDR): Leads to vision loss and presents
- Dot/blot hemorrhages/cotton-wool spots
- Intra-retinal hemorrhages/hard exudates
- Microvascular abnormalities (including microaneurysms, vascular malformation, and vascular tortuosity)
Proliferative diabetic retinopathy (PDR):
Sudden visual loss
- Neovascularization of the disk and retinal vessels
- Preretinal and vitreous hemorrhage
- Traction retinal detachment
B) Diabetic neuropathy
Peripheral neuropathy
- Numbness or reduced ability to feel pain
- Tingling or burning feeling
- Muscle weakness and difficulty walking
Autonomic neuropathy
- Nausea, vomiting, and loss of appetite
- Tachycardia
- Orthostatic hypotension
- Hypoglycemia unawareness
- Gustatory sweating (due to damage to a nerve which goes to a parotid gland)
- Urinary incontinence
- Constipation/uncontrolled diarrhea
- Gastroparesis
- Erectile dysfunction in men
- Retrograde ejaculation
- Vaginal dryness and other sexual difficulties in women
C) Diabetic nephropathy
- Often asymptomatic at diagnosis; progresses from microalbuminuria to more severe dysfunction
- Swelling of the feet and ankles
- Weakness/loss of appetite
- Upset stomach
- Insomnia and difficulty sleeping
- Confusion and trouble concentrating
Macrovascular:
A) Coronary heart disease (including silent MI): Is higher in patients with diabetes as compared to individuals with no diabetes. Patients with diabetes should be routinely monitored for cardiovascular risks, edema, dyspnea or orthopnea.
- Commonly asymptomatic
- ECG changes may indicate silent myocardial infarction
- Chest pain/pressure
- Pain in the jaw, neck, or epigastric area
- Dyspnea/orthopnea
- Paroxysmal nocturnal
- Edema
B) Peripheral vascular disease
- Intermittent claudication
- Pale/cool extremity
- Decreased hair growth in lower extremities
- Absent or decreased anterior tibial or dorsalis pedis pulses
- Poor capillary refill of toenails
- Ischemic gangrene
C) Cerebrovascular disease
Change in mental status (hypo or hyperglycemia; stroke)
Stroke may present as follows:
- Hemiparesis/hemisensory loss
- Dysarthria/aphasia
- Hemianopsia
- Monocular blindness
The Diabetic Foot
Usually, it is a combination of several factors, including:
- Diabetic neuropathy, trauma, and deformity, ischemia, callus formation, and edema
Diabetic foot disorders include:
- Foot ulcers, infections, ischemic necrosis, gangrene, callus formation, and Charcot neuroarthropathy
Differential Diagnosis
– Type 1 diabetes mellitus
– Type 2 diabetes mellitus
– Gestational diabetes mellitus (GDM)
– Maturity-onset diabetes of the young (MODY)
– Diabetes Secondary to:
- Pancreatic disease e.g. pancreatitis, pancreatectomy, neoplasia, cystic fibrosis
- Liver disease e.g. cirrhosis, hemochromatosis
- Hormonal disorders e.g. pheochromocytoma, acromegaly, Cushing syndrome, glucagonoma, hyperthyroidism, somatostatinoma, aldosteronoma
- Hereditary neuromuscular disease
- Lipodystrophy
- Insulin receptors disorders e.g. acanthosis nigricans syndrome, leprechaunism
- Genetic disorders e.g. trisomy 21, Klinefelter`s syndrome, Turner syndrome, Prader-Willi syndrome
- Drug or chemical induced glucose intolerance e.g. glucocorticoids, sympathomimetics, thiazide diuretics, phenytoin, pentamidine
Investigation and Workup

In asymptomatic individuals consider screening for diabetes if any of the following risk factors are present:
- Age ≥40 years (every 3 years)
- First-degree relative with type 2 diabetes
- Members of a high-risk ethnic group
- Hypertensive (≥140/90 mmHg)
- Cardiovascular disease
- Hyperlipidemia
- Sedentary lifestyle
- Overweight (BMI ≥25.0 kg/m²)/abdominal obesity
- Delivered a baby >4.5 kg (>9 pounds)
- History of gestational diabetes mellitus
- Previously found impaired glucose tolerance or impaired fasting glucose
- Polycystic ovary syndrome
- Psychiatric illness (e.g. schizophrenia)
- Acanthosis nigricans
Systematic screening:
Fasting plasma glucose (FPG)
- Usually first screening test
- Normal: <6.1 mmol/L (<110 mg/dL)
- Diabetes: ≥7.0 mmol/L (≥126 mg/dL)
2-hour plasma glucose in a 75-g oral glucose tolerance test (OGTT): indicated in
- When the FPG is 6.1 to 6.9 mmol/L (110-125 mg/dL)
- When FPG is 5.6 to 6.0 mmol/L (100-108 mg/dL) and ≥1 risk factors
- Impaired glucose tolerance is high (e.g. for individuals with risk factors)
- The test should be performed after an overnight fast of 8 to 14 hours and after at least 3 days of unrestricted diet (i.e. ≥150 g carbohydrate per day) and unlimited physical activity
Diagnostic criteria for diabetes

Guidelines for plasma glucose Levels for diagnosis of IFG, IGT and diabetes mellitus
Metabolic Syndrome
Metabolic Syndrome refers to a combination of metabolic derangements in which obesity and glucose impairment play a central role.
However, the diagnostic criteria are not universal and variance occurs among the governing organization e.g. International Diabetes Federation (IDF), World Health Organization (WHO), and National Cholesterol Education Program (NCEP) etc. There have also been attempts to unify the various criteria through a Harmonized definition.
Screening and Diagnostic Criteria for Gestational Diabetes Mellitus (GDM):
Screen all pregnant women for gestational diabetes mellitus:
- Low risk: Screen at 24 to 28 weeks gestation
- High risk: Screen at 20 weeks gestation
In all pregnant women: Measure FPG at the first prenatal visit (before 20 wks)
There are two approaches for GDM screening.
One-step approach:
- 75-g oral glucose tolerance test (75-g 2-h OGTT)
- Fasting plasma glucose level: ≥5.3 mmol/L (≥95 mg/dL)
- 1-hour plasma glucose level: ≥10.0 mmol/L (≥180 mg/dL)
- 2-hour plasma glucose level: ≥8.6 mmol/L (≥155 mg/dL)
- Interpretation: Two or more of the plasma concentrations must be met or exceed for a positive diagnosis on the OGTT
Two-step approach:
- Initially, screen with 50-g glucose challenge test (GCT)
- If PG is <7.8 mmol/L, then reassess in subsequent trimesters
- If 1h PG is between 7.8 to 10.2 mmol/L (140 to 180 mg/dL), should undergo (75-g 2-h OGTT)
- If 1h PG is ≥10.3 mmol/L, it is diagnostic of GDM
History (known or suspected DM):
- Family history of DM and its complications
- Personal history of other autoimmune disorders
- Risk factors e.g. hypertension/CVD/hyperlipidemia etc.
- Medications/immunization
- Physical activity
- Dietary intake such as; total calories, sugar, fats, frequency of meals
- Smoking, ethanol use
- Diarrhea, constipation
- Gestational diabetes
- Polyuria, polydipsia, nocturia, polyphagia, blurred vision, fainting, numbness, recurrent infections, skin ulcer
- Symptoms and/or signs of hypoglycemia:
- Palpitations, sweating, anxiety, hunger, tremor, confusion, seizures
- Symptoms and/or signs of acute hyperglycemia
- Nausea/vomiting, abdominal pain, shortness of breath, altered sensorium, fruity odor on breath, respiratory difficulty
Physical examination:
Should include assessment of
- Weight or body mass index: Overweight (BMI 25-29.9) or obese (BMI ≥30)
- Central obesity: Defined as waist circumference with ethnicity-specific values. If an individual is obese (BMI ≥30) waist circumference need not be measured
- Dilated retinal examination (Retinopathy)
- Conducted at the time of diagnosis of T2DM or 5 years after T1DM is diagnosed, then annual examinations should be performed
- Blood pressure of >130/80 mmHg considered hypertension in DM
- Foot exam: Look for skin ulcers, hammer or claw toes, Charcot foot, and dry skin (potential site for ulceration)
- Lower extremity examination: To seek evidence of peripheral neuropathy
- Peripheral Arteries:
Check for diminished pulsation. Ankle-brachial index test is helpful and compares BP measured at both ankles (ankle pressure) with both arms (brachial pressure) to assess for a gradient that might suggest the presence of peripheral artery disease (PAD)
Note: It is calculated by dividing the higher of the two ankle pressures by the highest systolic brachial pressure.
– Interpretation of ankle-brachial index
- Skin examination:
- Diabetic dermopathy: Roundish brown or purplish color, slightly-indented patches of skin
- Furuncles: Tender, pinkish-red, swollen nodule
- Carbuncles: Hot red nodule with visible layers of pus under the surface of the skin
- Candidiasis: Creamy white patches in the mouth or on the throat; skin rashes, patches, and blisters in the groin between fingers and toes and under the breasts; vaginal itching and irritation
- Necrobiosis lipoidica diabeticorum: 1 to 3 mm well-circumscribed erythematous papules or nodules; center of the rash are usually yellow
- Poorly healing abrasions or ulcerations
- Nail disease: e.g. Onychomycosis, ingrown toenails etc.
- Oral examination: For periodontal disease.
Laboratories
- Serum glucose level
- Random blood sugar ≥11.1 mmol/L
- Fasting blood sugar ≥7.0 mmol/L
- 2h postprandial in a 75 g OGTT ≥11.1 mmol/L
- Hemoglobin A1C≥ 6.5% (except in children, adolescents, pregnancy and type 1 diabetes)
Causes of high/low HbA1c levels
- Serum fructosamine:
- Normal values: 1.5-2.4 mmol/L when the serum albumin level is 50 gm/L
- Usually considered when HbA1C is not beneficial, low serum albumin levels will give low false results
- Immunologic markers
- Islet-cell autoantibodies (GAD-65): Present in >75% of patients with new-onset T1DM
- C-peptide assay (connecting peptide assay)
- C-peptide acts as a marker for endogenous insulin production
- Help distinguish T1DM from T2DM
- Absence of C-peptide indicates no beta cell function (likely T1DM)
- Urinalysis: Assess for glucosuria, ketonuria, infection
- Urinary albumin excretion
Diabetes is the most common cause of renal failure. Testing for albumin excretion rate is usually conducted 5 years after the diagnosis of T1DM or at the time of T2DM diagnosis, followed by annual assessment
Classification of abnormal renal albumin excretion rate (WHO)
- Fasting lipid profile
Performed at the time of diagnosis, and then every 1 to 3 years or more frequent testing is indicated if treatment for dyslipidemia is started
- LDL cholesterol target is <2.0 mmol/L or >50% reduction from baseline
Lipid targets for individuals with diabetes at high risk for CVD
- Serum thyroid-stimulating hormone (TSH)
- Recommended in T1DM due to higher prevalence of autoimmune thyroid disease (AITD) thyroid disorders
- ECG: Annual screening is usually recommended in all individuals >40 years or above, or with the duration of diabetes >5 years, or with HTN/proteinuria/vascular bruit/decreased pulses. Repeat ECG in patients at high risk for CV events
Investigations in common hyperglycemic states
Other investigations:
- Complete blood count
- BUN and serum creatinine
- Serum amylase and lipase
- Beta-hCG
- Chest X-ray
- Exercise stress test: As required for assessment of CAD risk
Screening for complications:
Begins 5 years after the diagnosis or at the time of diagnosis with T2DM. Annual assessment should be performed in all patients and includes:
- Macrovascular disease: Claudication, angina, and transient ischemic attack/stroke; bruits
- Retinopathy: Fundoscopy and visual acuity
- Nephropathy: Urinalysis for proteinuria and microalbuminuria; albumin/creatinine ratio
- Peripheral neuropathy: Paraesthesia, numbness, weakness
- Autonomic neuropathy: Postural hypotension, erectile dysfunction
- Foot examination: Arthropathy, calluses, infections
Treatment
- Canadian Diabetes Association (CDA): Blood glucose targets for diabetes
- American Association of Clinical Endocrinologists (AACE): Blood glucose targets for diabetes
- American Diabetes Association (ADA): Blood glucose targets for diabetes
NON-PHARMACOLOGICAL MANAGEMENT:
a) Diabetes self-management education (DSME):
- Ongoing process to facilitate knowledge, skills, and ability necessary for diabetes self-care
- Objectives are to support decision-making, self-care behaviors, problem-solving and coordination with the health care provider to improve adherence to the treatment, and its outcomes, and quality of life
b) Exercise: Improves glycemic control and strength, lipid profile, increases cardiorespiratory fitness and maintain weight.
Aerobic exercises:
- Recommended for a minimum of 2.5 hr/week, with >10min per session of moderate to vigorous-intensity, with no more than 2 consecutive days without exercise
Moderate exercises:
Examples of recommended exercises in patients who have 50-70% of maximum heart rate.
- Biking/brisk walking
- Swimming
- Dancing/raking leaves
- Water aerobics
Vigorous exercises:
Recommended in patients who have >70% of maximum heart rate
- Brisk walking up an incline
- Jogging/aerobics
- Hockey/basketball
- Fast swimming
- Fast dancing
Resistance exercises:
Weight-bearing or other forms of resistance exercises that require muscular strength conducted 3 times per week, in addition to aerobic exercise. Suggest beginning initially with instruction by an exercise specialist. Examples:
- Exercise with weight machines
- Weight lifting: Start with 1 set of 10-15 repetitions at moderate weight. Progress to 2 sets of 10-15 repetitions, then 3 sets of 8 repetitions at a heavier weight
Note: Conditions which may contraindicate certain types of exercise
- Severe autonomic/peripheral neuropathy
- Preproliferative or proliferative retinopathy
- Individuals with or at high risk of CVD
- Perform stress test before initiating exercise. Monitor glucose level before, during and after exercise and ingest carbohydrate if needed to prevent hypoglycemia
c) Nutritional behavioral management:
Is adapted to the individual’s lifestyle, culture, pharmacological management of DM and concomitant medical conditions
Weight Loss:
Gradual weight loss advised to obese individuals (especially T2DM) with reasonable, achievable short term goal such as 5 kg in 3 months
- Recommended caloric restriction for overweight
- Men = 1200-1800 kcal/day
- Women = 1000-1500 kcal/day
Carbohydrate (45-60% of energy): Consume low-glycemic-index foods (55 or less) in place of high-glycemic-index foods (70 or more) [Glycemic Index (GI): Rise in blood glucose level per each gram of carbohydrate in consumed food relative to glucose consumption; Glucose has 100 GI ].
Low-glycemic-index foods: Example beans, vegetables, most fruits (apples), whole grain wheat bread, rye, pita bread, high bran cereals, skim or 1% milk, chicken, turkey, fish, sweet potato, pasta, converted rice.
Sucrose: Intake of up to 10% of total daily energy (e.g. 50 to 65 g/day in a 2000 to 2600 kcal/day diet) is acceptable.
Fructose: Up to 60 g of added fructose in place of an equal amount of sucrose is acceptable.
Sugar alcohols: Intake of <10 g/day of sugar alcohols (maltitol, mannitol, sorbitol, lactitol, isomalt, and xylitol) is acceptable.
Protein (15-20% of energy): Recommended intake should be at least 0.86 g/kg/day. Preference is given to vegetable protein rather than the animal protein.
Fat (<35% of energy): Restrict saturated fats to <7% of total daily energy intake and restrict trans fat intake to a minimum. Limit polyunsaturated fat to <10% of energy intake. Monounsaturated fats are preferred instead of saturated fats. Include foods rich in polyunsaturated omega-3 fatty acids and plant oils.
Vitamin and mineral supplements: Balanced diet is sufficient source, except for
- Vitamin D: 10 μg (400 IU) supplementation is recommended in persons aged >50 years
- Folic acid: 0.4 mg to 1.0 mg/day folic acid prior to conception and during the early weeks of pregnancy is recommended
Alcohol: Ingestion may mask the symptoms of hypoglycemia, reduce hepatic production of glucose and impair an individual’s judgment
Alcohol avoidance: Advised in certain medical conditions such as severe hypertriglyceridemia, pancreatitis, advanced neuropathy, liver disease, prior history of alcohol abuse, pregnancy, and lactation.
Alcohol restriction:
- Two standard drinks per day or 14 drinks per week for men
- One standard drink/day or 9 drinks/week for women and lighter weight men
- One standard drink is equivalent to:
- 5oz/142 ml of wine (12% alcohol)
- 1.5oz/43 ml of spirits (40% alcohol)
- 12oz/341 ml regular strength beer (5% alcohol)
Smoking: Cessation of smoking and smoke-free environment is important in the overall management of diabetics to reduce cardiovascular risk.
Vaccination:
- An annual influenza vaccine is advised to reduce the risk of complications associated with influenza epidemics
- One-time pneumococcal revaccination is recommended for individuals >64 years old if the original vaccine was given >5 years prior
Ref: Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2008; 32:S1-S201.
d) Co-existing medical conditions
Hypertension:
- An intake of <100 mmol/day of sodium or 6g/day of sodium chloride (2.4 g/day sodium) is recommended (equivalent to <1.25 teaspoons of salt or 3 teaspoons of monosodium glutamate)
- Incorporate the ‘DASH Diet’ (Dietary Approaches to Stop Hypertension)
- Target blood pressure = systolic <130 mmHg / diastolic ≤80 mmHg
Dyslipidemia
- Restriction saturated fat to <7% and cholesterol to <200 mg/day of energy
- Omega-3 PUFA rich fish are encouraged at least once a week
Nephropathy
- Mild: Limit protein intake to 0.8-1.0 g/kg/day
- Overt nephropathy: Limit protein intake to 0.86 g/kg/day in adults
- In the presence of anemia, emphasize heme-iron (lean red meat) choices of protein while maintaining a 0.86 g/kg/day restricted intake
Neuropathy (to avoid or minimize gastropathy symptoms)
- Chew food thoroughly
- Small frequent low-fat meals
- Increase intake of foods of higher liquid consistency
PHARMACOLOGICAL THERAPY:
If the patient’s glycemic target is not achieved after 4 weeks of medical nutrition therapy, pharmacologic therapy is indicated.
Antihyperglycemic agents often used are; insulin therapy and oral glucose-lowering agents and more recently injectable incretin agents.
Type 1 diabetes mellitus (T1DM)
Basal-bolus insulin regimens (e.g. multiple daily injections or continuous subcutaneous insulin infusion) are the regimens of choice for all adults with T1DM.
Insulin regimens should be customized to the individual’s
- Treatment goals
- Lifestyle/general health
- Diet/age
- Motivation
- Hypoglycemia awareness status
- Ability for self-management
All individuals with type 1 diabetes should be counseled about the risk, prevention, and treatment of insulin-induced hypoglycemia. In some cases, you may see a honeymoon period.
Insulin regimens:
Total daily dosing (TDD) for 70 kg person
TDD = 0.5 x 70 = 35 units approximately
Basal-bolus regimens/multiple daily injections (MDI)
- Bolus (60% of TDD): Short or rapid-acting insulin before each meal
- Basal (40% of TDD): Intermediate or long-acting insulin at bedtime or intermediate insulin split twice per day
CSII (continuous subcutaneous insulin infusion)
Fast-acting insulin is used and multiple basal infusion rates can be programmed according to requirement.
- Initially calculate dose as total pre-pump dose “x” and multiply it by 80% = 0.8x
- Start the basal rate at 50% of that (0.4x) and divide it by 24 hours = (x/60) units/hr
- Divide the total carbohydrates/day into 3 equal meals and give 50% of the pre-pump dose as three equal boluses of (0.4x/3) = (x/7.5) units/meal
- If no prior insulin history is known, estimate total dose by 0.44 times the weight in kilograms
Available Insulin Preparations
Type 2 Diabetes Mellitus
Once T2DM established, initiate and maintain lifestyle modification. It is recommended to begin oral antihyperglycemic agents for patients with T2DM; who do not achieve glucose control with diet and exercise alone as per recommendations.
Hyperglycemia management in type 2 diabetes
GLP-1 receptor agonist
Exenatide:
Currently approved as adjunctive therapy for use in combination with sulfonylurea, metformin, or both.
- Indicated in combination with metformin, and/or sulfonylurea to improve glycemic control in patients with T2DM, when maximally tolerated doses of these oral therapies in addition to diet and exercise do not provide adequate glycemic control
Liraglutide:
- Once-daily dosing in patients with T2DM in combination with metformin therapy or combined metformin and sulfonylurea therapy, when maximally tolerated doses of these oral therapies in addition to diet and exercise do not provide adequate glycemic control
Combined formulations are also available:
- Rosiglitazone + Metformin
- Rosiglitazone + Glimepiride
- Sitagliptin + Metformin
Newer therapeutic agents:
Amylin analogue
Pramlintide: (available in Canada now)
- Adjunctive treatment with mealtime insulin in T2DM; in patients who have failed to achieve desired glucose control despite optimal insulin therapy
Bromocriptine mesylate-(available in the USA):
- FDA-approved novel treatment for type 2 diabetes as an adjunct to diet and exercise to improve glycemic control in adults with T2DM
Gestational diabetes mellitus (GDM):
Once diagnosed monitor four times daily.
Goals:
- Fasting: 3.8-5.2 mmol/L (68-94 mg/dL)
- 1 hour postprandial: 5.5-7.7 mmol/L (99-139 mg/dL)
- 2 hour postprandial: 5.0-6.6 mmol/L (90-119 mg/dL)
Patients with GDM are high risk patients for type 2 diabetes, should be re-evaluated 6 wks and 6 months after delivery.
Diabetes sick-day
During illness or infection blood glucose level often rises even though the person is not eating.
Goals for sick-day management
- Prevention of hyperglycemia and hypoglycemia
- Maintenance of hydration
Sick-day guidelines
- Always take insulin or diabetes medication
- Take at least the usual dosage of insulin
- Sometimes an increase in the dosage of insulin is required
- Monitor blood glucose and urine ketones every 4 hrs
- Drink plenty of fluids, 6-8 oz of fluid every hour is recommended
- If unable to eat, drink fluids that contain carbohydrates (e.g. fruit juices, regular soda)
- Try to eat usual amount of carbohydrate (CHO), may be divided into smaller meals and snack, if having difficulty eating, eat or drink 15 g of carbohydrate every hour or 45-50 g of carbohydrate every 3-4 hrs
Metabolic syndrome
- Treat underlying causes (overweight/obesity and physical inactivity)
- Intensify weight management
- Increase physical activity
- Treat lipid and non-lipid risk factors
- Treat hypertension
- ACE inhibitor or ARB (angiotensin receptor blocker) is usually prescribed for patients with diabetes
- Treat elevated triglycerides
- Statin if predominantly high LDL
- Fibrate if predominantly high triglycerides
- Use antiplatelet therapy (e.g. aspirin) for patients with CAD, stroke, PAD unless otherwise contraindicated
Acute complications of diabetes mellitus:
- Hypoglycemia
- Diabetic ketoacidosis (DKA)
- Hyperosmolar hyperglycemic state (HHS)
Hypoglycemia
Indications for hospitalization hypoglycemia:
- If induced by sulfonylurea
- Deliberate drug overdose
- Coma, seizure
- Persistent neurological changes
Management:
Mild to moderate hypoglycemia:
15 gm of fast-acting carbohydrate can be given in any one of the following ways as follows:
- Three or four commercially prepared glucose tablets
- Regular soft drink or fruit juice (120-180 ml)
- Hard candies or 6 lifesavers (1 = 2.5 g of carbohydrate)
- 3 teaspoons) or 3 packets of table sugar dissolved in water
- 15 mL (1 tablespoon) of honey
Retest blood glucose levels after ~15 minutes, and administer another 15 g glucose if BG remains <4.0 mmol/L (72 mg/dL).
Note: 15 gm of carbohydrate will produce a blood glucose increment of approximately 2.1 mmol/L (38 mg/dL) in 20 minutes.
Severe hypoglycemia in a conscious person:
- Oral ingestion of 20 g of carbohydrate, preferably as glucose tablets or equivalent.is administered
- Retest blood glucose levels after ~15 minutes, and retreat with another 15 g of glucose if the blood glucose level remains <4.0 mmol/L (72 mg/dL)
Note: 20 g oral glucose dose will produce a BG increment of approximately 3.6 mmol/L (65 mg/dL) at 45 minutes.
Severe hypoglycemia in an unconscious person or unable to have oral intake at home:
- Administer glucagon 1mg, either SC or IM in the deltoid or anterior thigh
- This should produce blood glucose rise from 3.0-12 mmol/L (54-216 mg/dL) within 60 minutes
- Once patient regains consciousness regains, high carbohydrate source snack is provided provided
Severe hypoglycemia in an unconscious person with IV access (usually hospital setting):
- Administer glucose 10-25 g (20-50 ml of D50W) IV over 1 to 3 minutes
- Assure patency of IV line because hypertonic solution of 50% DW is very irritable to the veins
Diabetic ketoacidosis (DKA):
Potentially fatal complication occurs in up to 5% of Type1 DM patients.
Risk Factors:
- Interruption in insulin therapy
- Sepsis, trauma, and MI, etc.
- Pregnancy
Indications for hospitalization with DKA include:
- Plasma Glucose Levels: 14.0 mmol/L (>250 mg/dL)
- Arterial PH: < 7.30
- Serum Bicarbonate: <15 mEq/L
- Moderate ketonemia or ketonuria
Management:
- Oxygen and airway management as needed
- Immediate IV access
- Hydration: as follows
0.9% NaCl IV usually 1-2 liters should be given rapidly (if cardiac functions are normal) followed by 0.5 to 1 liter/hr until vital signs are stabilized, and urine output has been maintained.
Hypotonic saline (0.45%) in patients with severe hypernatremia (>150mEq/L).
Replenish total body water deficit by giving:
- High or normal sodium levels: 0.45% saline at 150-500 ml/hr
- Low sodium levels: 0.9% NaCl saline 150-500 ml/hr
Note: Avoid the risk of cerebral edema, do not exceed the change in osmolality >3 mmol/kg/hr.
The goal is to replace approximately 50% of the total body water (TBW) deficit in the first 8 hours and the remainder in the subsequent 16 hours.
Formula: Total body water deficit (L) = 0.6 x wt (kg) x [1-140/serum sodium]
Potassium replacement
- Potassium deficit should always be assumed. Insulin therapy should be held if K+ is <3.3 mmol/L, and potassium should be replaced immediately at the rate of 40 mmol/hr in a solution of one liter of 0.9% normal saline
- 10-20 mEq/hr of potassium should be added to the 2nd or 3rd liter of fluid unless the patient has hyperkalemia (>5.5 mmol/L or ECG evidence) or renal failure or oliguria
Monitoring
- Blood glucose levels hourly and serum electrolytes every 1-2 hours
- Continuous ECG monitoring is required
Insulin therapy:
Is necessary for resolution of the ketoacidosis, can be withheld initially in patients with marked hypokalemia.
- Immediately give IV bolus of 10-15 unit of Insulin, followed by continuous infusion of regular insulin at an initial rate of 5-10 units/hr. Dilute 100 units in 100 ml of 0.9% saline, infuse at the rate of 10 ml/hr will deliver 10 units/hr
- Target: Decrease of 2.8-4.1 mmol/hr (50-75 mg/dL/hr) of blood glucose levels
Do’s And Don’ts:
- If insulin resistance is suspected then hourly dose of regular insulin should be increased by 50-100%
- Avoid reduction at rate >5.5 mmol/L/hr (100 mg/dL/hr) due to osmotic encephalopathy
- Maintain insulin infusion over 1-2 units/hr, and continue till patient clinically improved. Once oral intake resumes insulin can be given SC
Dextrose:
- Administer Dextrose 5% in IV fluid once plasma glucose level decrease to 14.0 mmol/L (250 mg/dL). Decrease insulin rate to 0.05 units/kg/hr to avoid hypoglycemia
Bicarbonate
- Serum bicarbonate levels and closure to anion gap is a reliable parameter to evaluate metabolic recovery
- Bicarbonate therapy is considered in shock or coma, severe acidosis (pH <7.0), plasma Bicarbonate (<5 mEq/L), acidosis induced cardiac or respiratory dysfunction, and severe Hyperkalemia
- Sodium bicarbonate, 50 mEq/L of 200 ml D5W can be given over 1 hr, can be repeated every 1-2 hrs until pH≥7.0
Phosphate and magnesium
- Stores are depleted but are not replaced if the patients resume oral intake. However, magnesium is replaced if the patient is at risk of ventricular arrhythmias
Antibiotics
Should be started promptly if there are documented infections.
- Empiric broad-spectrum antibiotics can be considered if the patient is in sepsis
Complications of DKA requiring immediate recognition and prompt treatment:
Lactic acidosis:
Despite of optimal DKA management, some patients may exhibit persistent anion gap with refractory metabolic acidosis, due to prolonged dehydration, shock, and sepsis or tissue hypoxia.
Management requires the following:
- Adequate volume replacement
- Control of sepsis
- Liberal and judicious use of bicarbonates
Arterial thrombosis results in stroke, MI or ischemic limb.
- Routine anticoagulation is not recommended
- Treatment guided by clinical scenario
Cerebral edema may occur from over rehydration, especially in children, and should be quickly recognized (headache, papilledema, altered sensorium); brain CT scan may assist diagnosis.
- Treat immediately by IV mannitol
Rebound ketoacidosis may result from premature discontinuation of IV insulin or inadequate SC doses of insulin.
Hyperosmolar hyperglycemic state (HHS):
Usually occurs in patients with type 2 diabetes mellitus and has no or little ketosis.
Indications for hospitalization HHS includes:
- Plasma Glucose Levels: 22.1 mmol/L (≥400 mg/dL)
- Serum osmolality: >320 mmol/kg
- Impaired mental status
Management:
- Oxygen and airway management as needed
- Immediate IV access
- Hydration
- Do not exceed the change in osmolality >3mmol/kg/hr
- The goal in fluid administration is to replace approximately 50% of the total body water (TBW) deficit in the first 8 hours and the remainder in the subsequent 16 hours
Potassium replacement
- Potassium deficit should always be assumed. Insulin therapy should be held if K+ is <3.3 mmol/L, and potassium should be replaced immediately at the rate of 40 mmol/hr in a solution of one liter of 0.9% normal saline
- 10-20 mEq/hr of potassium should be added to the 2nd or 3rd liter of fluid unless the patient has hyperkalemia (>5.5 mmol/L or ECG evidence) or renal failure or oliguria
Monitoring
- Blood glucose levels hourly and serum electrolytes every 1-2 hours
- Continuous ECG monitoring is required
Insulin therapy
Not critical for the treatment of HHS as compared to hydration which should always precede insulin administration.
- Generally, insulin use is recommended to reduce plasma glucose levels
Dextrose
- Administer Dextrose 5% in IV fluid once plasma glucose level decrease to 14.0 mmol/L (250 mg/dL), to avoid hypoglycemia
Seizure control
- Requires normalization of osmolality and glucose; standard antiepileptics may be ineffective.
- Avoid phenytoin, which inhibits the release of endogenous insulin and has been associated with HHS
Bicarbonate
- One ampoule (50 mmol) of sodium bicarb in 200 ml of D5W over 1 hr, repeated every 1-2 hrs, until pH ≥7.0 is considered in adults with shock or who are having pH ≤7.0
- Watch for delayed metabolic acidosis and hypokalemia
Phosphate and magnesium
- Stores are depleted but are not replaced if the patients resume oral intake. However, magnesium is replaced if the patient is at risk of ventricular arrhythmias
Antibiotics
Should be started promptly if there is documented infections.
- Empiric broad-spectrum antibiotics can be considered if the patient is in sepsis
Complications of HHS include:
Thromboembolic events, cerebral edema, adult respiratory distress syndrome, and rhabdomyolysis.
Management of chronic complications of Diabetes Mellitus
(i) Microvascular complications include:
- Diabetic retinopathy
- Diabetic neuropathy: Includes peripheral and autonomic neuropathy, as briefly summarized in the tables
- Diabetic nephropathy
(ii) Macrovascular
Coronary heart disease (CHD), stroke, and peripheral vascular disease (PVD) account for 80% of deaths in patients with diabetes.
- Cardiovascular risk should be assessed and treated aggressively
- Cigarette smoking should be discouraged
- Good hyperglycemic control after acute MI is beneficial
- In peripheral vascular disease (PVD) similar goals are to be achieved as described above for (CHD)
- Antiplatelet agents are recommended for secondary prevention in patients with stroke or heart disease and may have modest benefit in primary prevention in high-risk vascular patients (Framingham >20%-excluding diabetes)
- Exercise and agents such as pentoxifylline 400 mg TID and cilostazol 100 mg BID (the latter not currently available in Canada) may be helpful in intermittent claudication
(iii) The Diabetic Foot:
Proper foot care is advised to avoid problems, which includes; bathing, drying and lubricating without leaving the moisture to accumulate between the toes. Wear closed toe shoes which fit well, and trimming toenails straight across and file sharp corners.
Mild to moderate ulceration
- Rest, elevation and relief of pressure are essential
- Oral antibiotics should be started immediately e.g. amoxicillin/clavulanic acid 800 mg PO BID; cloxacillin 500 mg QID, first-generation cephalosporin or clindamycin are recommended
- If cellulitis does not respond with oral antibiotics IV should be started
Moderate to severe ulceration
- Infections require hospitalization and IV antibiotics
- Debridement may be required; both aerobic and anaerobic cultures are sent
- Empiric antimicrobials are given intravenously and the coverage is tailored according to response and cultures, agents like clindamycin, vancomycin, and fourth generation cephalosporin’s, ampicillin/sulbactam may be considered
Moderate to severe with ischemia or significant local necrosis
- Determine the presence of bone involvement and peripheral vascular disease
- IV antibiotics, surgical debridement, bed rest, cultures are the mainstay of treatment
- Presence of osteomyelitis requires 6-12 week of IV Antibiotics
- Localized or generalized ulcers with gangrene require amputation
Additional information in patients with or without diabetes who are admitted and managed in the hospitals.[/cq_vc_tab_item][cq_vc_tab_item tabtitle=”Medication Dose”]MEDICATIONS:
Sulfonylurea, first-generation agents
- Chlorpropamide
- Tolazamide
- Tolbutamide
Sulfonylurea, second-generation agents
- Glimepiride
- Glipizide
- Glyburide
Meglitinide drugs
- Nateglinide
- Repaglinide
Mechanism
- Blocks ATP-dependent potassium channels in the β-cell membrane → Depolarize the β-cell → Facilitating calcium entry through calcium channels → Increased calcium influx induces insulin secretion from the pancreatic beta cells
- Decrease in serum glucagon level
- Enhances peripheral utilization of glucose
- Decrease in hepatic insulin degradation and gluconeogenesis
- First generation sulfonylureas are differed with second-generation in their pharmacokinetics and lower dosage
- Meglitinides do not contain sulfur in their structure and have a rapid onset and short duration of action as compared to sulfonylurea
Dose:
Sulfonylurea, first-generation agents
Chlorpropamide
- 100-250 mg PO once daily; may titrate by 50-125 mg/day at 3-5-day intervals; Max. 500 mg/day
Tolazamide
- 100-250 mg PO once daily; with breakfast or the first main meal of the day; may increase dose by 100-250 mg/day every week if needed; Max. 1 g/day
Note: If >500 mg/day; divide in 2 doses over 24 hrs
Tolbutamide
- 1-2 g/day PO in 2-3 divided doses; usual 500-3000 mg/day; maintenance dose of >2g/day seldom required
Sulfonylurea, second-generation agents
Glimepiride
- 1-2 mg PO once daily; with breakfast or the first main meal; may increase dose by 1-2 mg/day every 1-2 wks; usual 1-4 mg PO once daily; Max. 8 mg/day
Dose in Renal Impairment: ClCr <22 mL/minute à Start 1 mg PO once daily; may increase dose slowly
Glipizide
- 5 mg PO once daily; may titrate dose by 2.5-5 mg/day as needed at least with intervals of several days; Max. 15 mg/dose and 40 mg/day (divide dose if >15 mg/day); for extended-release tablet maximum dose is 20 mg/day
Dose in Hepatic Impairment: Start with 2.5 mg/day
Glyburide
- 2.5-5 mg PO once daily; may titrate by 2.5 mg/day every week; Max. 20 mg/day in divided doses
Meglitinide Drugs
Nateglinide
- Initial and maintenance 120 mg PO TID before meals; 60 mg PO TID in patients who are near HbA1c goal; can be given alone or in combination with metformin or a thiazolidinedione
Repaglinide
- HbA1c <8% or Not treated previously → Start 0.5 mg PO with each meal; usual 0.5-4 mg
- HbA1c is ≥8% or Treated previously → Start 1-2 mg PO with each meal; usual 0.5-4 mg
Note: May titrate dose by double up to 4mg /dose; to a Maximum dose of 16 mg/day. At least lapse of one week is required to reassess the glucose levels
Dose in Renal Impairment: ClCr 20-40 mL/minute → Initial 0.5 mg before each meal, titrate carefully; ClCr <20 mL/minute → Use not established
Dose in Hepatic Impairment: conservative doses should be used
- Metformin
Mechanism
- Decreases hepatic glucose production, decreasing intestinal absorption of glucose and improves insulin sensitivity (increases peripheral glucose uptake and utilization)
Dose
Metformin
Immediate-release form
- 500 mg PO BID or 850 mg PO daily; may increase by 500 mg/wk or 850 mg/every other week; Max of 2550 mg/day in 3 divided doses
Extended-release form
- 500 mg PO daily; Usual 1-2 gram PO daily; May increase dose 500 mg/day every week to max 2g/day. Always use divided doses if giving >2000 mg/day for better tolerance
Concomitant with insulin therapy
- Start Metformin 500 mg PO daily; may increase by 500 mg/day weekly until adequate control is achieved; Max 2000 mg/day
- Miglitol
- Acarbose
Mechanism
- Competitive inhibitor of pancreatic α-amylase and intestinal brush border α-glucoside hydrolase → which results in delayed digestion/absorption of carbohydrates → leading to post-meal lowering of glucose, delaying glucose absorption.
Dose:
Miglitol
- 25 mg/day PO TID at the start of each meal; may increase to 50 mg PO TID at least after 4-8 weeks and to 100 mg TID after 3 months, if required; Max. 300 mg/day
Acarbose:
- Initial 25 mg PO TID at the start of each meal; usual 150-300 mg/day PO TID; may increase dose every 4-8 weeks prn; Max. in ≤60kg: 150 mg/day in 3 divided doses, >60 kg: 300 mg/day in 3 divided doses
Insulin Sensitizers (Thiazolidinediones)
- Pioglitazone
- Rosiglitazone
Mechanism
Increases insulin sensitivity without increasing pancreatic insulin secretion
- An agonist for peroxisome proliferator-activated receptor-gamma (PPARgamma) → found in key target tissues for insulin action → activate nuclear PPARgamma receptors → this influences the production of a number of gene products involved in glucose and lipid metabolism → lowers blood glucose
Dose:
Pioglitazone
- 15-30 mg/day PO once daily; may increase up to 45 mg/day; Max. 45 mg/day
Rosiglitazone
- Initial 4 mg/day PO once daily or in 2 divided doses; if inadequate response after 8-12 weeks the dose may be increased to 8 mg in 2 divided doses; Max. 8 mg/day
Note: Adjunct with a sulfonylurea, maximum dose is 4 mg/day
Dipeptidyl peptidase IV inhibitors
- Sitagliptin
- Saxagliptin
- Linagliptin (available now in Canada)
Mechanism
Inhibits dipeptidyl peptidase IV (DPP-IV) enzyme resulting in
- Prolonged active incretin levels
- Incretin hormones regulate glucose homeostasis by
- Increasing insulin synthesis and release from pancreatic beta cells
- Decreasing glucagon secretion from pancreatic alpha cells
Decreased glucagon secretion results in decreased hepatic glucose production.
Dose:
Sitagliptin
- 100 mg PO once daily; Max 100 mg/day
- 75-85% renally excreted
Dose in Renal Impairment: ClCr 15 to <50 mL/min → 50 mg PO once daily; ClCr <30 mL/min → 25 mg PO once daily
Saxagliptin
- 5 mg PO once daily
- Approximately 50% renal excretion
Dose in Renal Impairment: ClCr 15-50 mL/min → 2.5 mg PO once daily; end-stage renal disease → not recommended
Linagliptin
- 5 mg PO daily with or without food
- Minimal renal excretion
Glucagon-Like Peptide-1 (GLP-1) Receptor Agonist
- Exenatide
- Liraglutide
Mechanism
Activates glucagon-like-peptide-1 (GLP-1) receptor, this
- Increases insulin secretion and B-cell growth/replication
- Decreases inappropriate glucagon secretion
- Delay gastric emptying
Doses:
Exenatide:
- Initial 5 mcg SC BID; within 60 minutes prior to meals; may increase to 10 mcg SC BID after 1 month, if needed
Note: Give at least an interval of 6 hours between dosing and do not take after a meal.
Liraglutide:
- Start 0.6 mg SC daily x 1 week at least; then increase to 1.2 mg SC daily; after at least 1 week may increase to 1.8 mg SC daily as needed to achieve maximum effect
TYPES OF INSULIN:
Rapid-acting Insulin
- Insulin lispro
- Insulin aspart
- Insulin glulisine
Short-acting
- Regular insulin
Intermediate-acting
- NPH insulin (NPH = Neutral Protamine Hagedorn, also known as isophane insulin)
Long-acting, subcutaneous
- Insulin detemir
- Insulin glargine
Combinations
- 50/50 (50% NPH, 50% regular)
- 70/30 (70% NPH, 30% regular)
- 70/30 (70% protamine aspart, 30% aspart)
- 75/25 (75% protamine lispro, 25% lispro)
- 50/50-(50% protamine lispro, 50% lispro)
Mechanism
Insulin acts via specific membrane-bound receptors on target tissues.
- Enhanced peripheral glucose uptake and protein synthesis
- Inhibits hepatic glucose production, lipolysis, and proteolysis
Insulin increases the cellular permeability of several electrolytes, such as
- Potassium, magnesium, and phosphate
Insulin activates sodium-potassium ATPase; this promotes the intracellular movement of potassium.
Rate of absorption, onset, and duration of activity may be affected by:
- Site of injection, exercise, lipodystrophy, local blood supply, temperature
Dose:
Rapid-acting Insulin: Duration effect of Rapid-acting Insulin is <5 hours
Insulin Lispro; Insulin Aspart; Insulin Glulisine
- Usual total insulin requirement 0.5-1 units/kg/day SC divided in 3-4 doses
- Give <15min before meals or within 20 min after starting a meal
- IV infusion: Diluted in NS or D5W to concentrations of 0.025-2 units/mL. Stable for 48 hours at room temperature
Dose in Renal Impairment:
- Insulin Lispro: ClCr 10-50 → Decrease dose 25%; ClCr <10 → Decrease dose 50%; Dialysis → No supplement
- Insulin Aspart; Insulin Glulisine: Decrease the dose
Short-acting Insulin: Duration effect of Regular Insulin is ~5-8 hours
Regular Insulin:
- Usual insulin requirement 0.5-1 units/kg/day SC divided in 2-3 doses. Give <15min before meals or within 20 min after starting a meal
- IV infusion: Diluted in NS or D5W to concentrations of 0.025-2 units/mL
Dose in Renal Impairment:
- ClCr 10-50 → decrease dose 25%; ClCr <10 → decrease dose 50%; Dialysis → no supplement
Diabetic ketoacidosis/Hyperosmolar hyperglycemic state
- IV Bolus: 0.1 units/kg bolus (optional)
- IV Infusion: 0.1-0.14 units/kg/hour.
Note: If no IV bolus was administered, patients should receive a continuous infusion of 0.14 units/kg/hour
- SC/IM: 0.4 units/kg bolus (0.2 units/kg as an IV and 0.2 units/kg as SC or IM); then 0.1 units/kg given every hour SC or IM
Hyperkalemia
- IV: 10 units IV regular insulin mixed with 50 mL D50W given over 15-30 minutes OR50 mL D50W over 5 minutes followed by 10 units regular insulin IV push over seconds (in imminent cardiac arrest)
Intermediate-acting Insulin: Duration effect of NPH insulin is ~10-18 hours
NPH insulin:
- Usual total insulin requirement 0.5-1 units/kg/day SC (divided in 2-3 doses). Give <15min before meals or within 20 min after starting a meal
Dose in Renal Impairment:
- ClCr 10-50 → Decrease dose 25%; ClCr <10 → decrease dose 50%; Dialysis → no supplement
Long-acting Insulin: Duration effect of Insulin Detemir is ~16-23.2 hours; and Insulin Glargine is up to 24 hours
Insulin Detemir
- Type 1 DM: Start 0.5-1 units/kg/day SC in divided dose; usual dose 0.5-1.2 units/kg/day SC in divided doses
- Type 2 DM: Start 0.1-0.2 units/kg SC (every evening) or 10 units SC every evening or divided into 2 doses; may increase dose by 2 units/day every third day until glycemic targets achieved
Insulin Glargine
- Type 1 DM: Start 0.5-1 units/kg/day SC in divided dose; usual dose 0.5-1.2 units/kg/day SC in divided doses
- Type 2 DM: Initial 10 units SC daily at bed time; may increase dose by 2 units/day every third day until glycemic targets achieved
Newer therapeutic options
- Pramlintide (available in the USA not in Canada)
Mechanism
Decreases the rise of postprandial plasma glucose by
- Prolonging of gastric emptying time
- Suppresses postprandial glucagon secretion
- Promotes satiety leading to reduction of caloric intake and potential weight loss
Doses:
Note: When initiating pramlintide, decrease current insulin dose by 50% to avoid hypoglycemia
- Type 1 DM: Initial 15 mcg SC immediately prior to meals; may increase by 15 mcg SC every third day to target dose of 30-60 mcg, if needed and nausea is tolerable
Note: Consider Pramlintide discontinuation if 30 mcg dose is not tolerated
- Type 2 DM: Initial 60 mcg SC immediately prior to meals; may increase to 120 mcg SC after 3-7 days, if needed and nausea is tolerable
Note: If nausea occurs at 120 mcg dose, decrease dose to 60 mcg
- Bromocriptine
Mechanism
- Exact mechanism is not known
- Activates postsynaptic dopamine receptors in the striatum and substantia nigra
- Bromocriptine also suppresses growth hormone production and decreases prolactin secretion
- Results in the reversal of insulin resistance and decreases in glucose production
- It decreases fasting and postprandial hyperglycemia without increasing insulin levels
Doses:
- Type 2 DM: Start 0.8 mg PO every morning; may increase by 0.8 mg every week as tolerated to the maximum dose of 4.8 mg/day; usual dose 1.6-4.8 mg PO every morning
[/cq_vc_tab_item][/cq_vc_tabs]
Clinical Trials
- ACCORD (GLUCOSE) STUDY: Effects of Intensive Glucose Lowering in Type 2 Diabetes
- ACCORD (LIPID) STUDY: Effects of Combination Lipid Therapy in Type 2 Diabetes Mellitus
- ACCORD (BLOOD PRESSURE) STUDY: Effects of Intensive Blood-Pressure Control in Type 2 Diabetes Mellitus
- ADVANCE STUDY: Action in Diabetes and Vascular Disease: Intensive Blood Glucose Control and Vascular Outcomes in Patients with Type 2 Diabetes
- VADT STUDY: Glucose Control and Vascular Complications in Veterans with Type 2 Diabetes
- Origin Trial: Basal Insulin and Cardiovascular and Other Outcomes in Dysglycemia
Pipeline Agents
- Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycemic control with metformin
- Insulin degludec, an ultra-long-acting basal insulin, once a day or three times a week versus insulin glargine once a day in patients with type 2 diabetes
- Dose-dependent effects of the once-daily GLP-1 receptor agonist lixisenatide in patients with Type 2 diabetes inadequately controlled with metformin
Physician Resources
1. Tips for patient care
Risk factor management:
- Encourage the use of medic Alert bracelet or the equivalent
- Counsel patients, about signs and symptoms of hypoglycemia and strategies to avoid them, particularly those on insulin
- For those at risk of severe hypoglycemia, caregivers/support persons should be taught how to administer glucagon by injection
- Screen for foot care/infection; if suspect infection treat aggressively
- Patient should be made aware of the potential risk of atherosclerosis and vascular risk and should be encouraged to comply with medications, discontinue smoking (if applicable), and address other vascular risk factors
Monitoring:
- Monitor blood sugar and BP, body weight and the presence of complications
- Hemoglobin A1C measurement
- Measure twice per year in patients with good glycemic control
- Every 3 months in most patients: Those whose therapy has changed, or who are not meeting glycemic goals
- Compare meter results with laboratory measurements at least annually; or when indicators of glycemic control do not match meter
- Review home monitoring records and note the occurrence and frequency of hypoglycemia
- Avoid premature switch to SC insulin and/or discharge, this may result in high recurrence and readmission rates
Medications:
- Advise the patient to establish a routine for taking their medications
- Patients with diabetes should be taught how to match insulin to carbohydrate intake
- Use of premixed insulin as an alternative to mixing insulin minimizes dose errors
- Before initiating insulin pump therapy, multiple daily injections (MDI) should be discontinued and allow sufficient time for intermediate or long-acting insulin to taper off
- Adherence to treatment should be assessed at each visit
- Monitor patients regularly to adjust medications as required
- Consider concurrent risk factors and disease states with the prescribed therapy
- Evaluate cost and affordability/insurance coverage when prescribing; costly agents could impact on compliance/adherence
Social and Stress factors:
- Ensure patient and family are well informed about the disease and its treatment
- Individuals with diabetes should be regularly screened for subclinical psychological stressors
- Dietary counseling by a registered dietitian is helpful
- Activities (such as physical, mental):
- Encourage an active lifestyle and regular exercise
- Advice patients to keep themselves mentally active
- Include family or social support in lifestyle modification
Diabetes and Pregnancy:
Prior to the conception and during pregnancy:
- Discontinue oral antihyperglycemic agents, and switch the patient to insulin
- Prescribe folic acid at a dose of 1-4 mg/day before conception until 13 weeks of gestation
- Gestational DM may be associated with an increased risk of:
- Childhood obesity and diabetes in the offspring
- Increased risk for future diabetes in the mother
- Poor diabetic control at the time of conception increases the risk of:
- Spontaneous abortion/prenatal mortality
- Morbidity/congenital malformations
- Both retinal and renal disease may worsen significantly during pregnancy
Alerts:
- Risk of stroke is ~2-fold higher in diabetic adults compared to those without diabetes
- Patient should recognize the early signs and symptoms of hypoglycemia and hyperglycemia
- Individuals with diabetes should be aware that alcohol consumption can lead to delayed hypoglycemia
- Sulfa allergy: Caution with sulfonylureas use
- Absence of fever and/or leukocytosis does not rule out infection
- If abdominal pain persists after DKA treated, reassess to rule out other pathological cause
2. Tables and Scales:
References
Core Resources:
- American Association of Clinical Endocrinologists (AACE) medical guidelines for clinical practice for developing a diabetes mellitus comprehensive care plan, Endocr Pract 2011;17 (S2):1-53
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34:S62-S69
- Compendium of Pharmaceuticals and Specialties (CPS). Canadian Pharmacist Association. Toronto: Webcom Inc. 201
- Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2008;32:S1-S201
- Day RA, Paul P, Williams B, et al (eds). Brunner & Suddarth’s Textbook of Canadian Medical-Surgical Nursing. 2nd ed. Philadelphia: Lippincott Williams and Wilkins; 2010
- Foster C, Mistry NF, Peddi PF, Sharma S, eds. The Washington Manual of Medical Therapeutics. 33rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010
- Goldenberg RM, Cheng AYY, Punthakee Z, Clement. Use of Glycated Hemoglobin (A1C) in the Diagnosis of Type 2 Diabetes Mellitus in Adults (Position Statement) Can J. Diabetes 2011; 235: 247-249
- Gray J, ed. Therapeutic Choices. Canadian Pharmacists Association. 6th ed. Toronto: Webcom Inc. 2011
- Grundy SM, Cleeman JI, Bairey Merz CN, et al, for the Coordinating Committee of the National Cholesterol Education Program. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines.Circulation. 2004; 110:227-239
- International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327-1334.
- Katzung BG, Masters SB, Trevor AJ, eds. Basic and Clinical Pharmacology. 11th ed. New York: McGraw-Hill; 2009
- Leiter LA, Fitchett DH, Gilbert RE, Gupta M, et al. Cardiometabolic Risk in Canada: A Detailed Analysis and Position Paper. Canadian Journal of Cardiology 27 (2011) e1-e33
- Longo D, Fauci A, Kasper D, et al (eds). Harrison’s Principles of Internal Medicine. 18thed. New York: McGraw-Hill; 2011
- McPhee SJ, Papadakis MA, eds. Current Medical Diagnosis & Treatment. 49th ed. New York: McGraw-Hill; 2010
- Medical Guidelines for Clinical Practice for the Management of Diabetes Mellitus 2007; American Association of Clinical Endocrinologists
- Pagana KD, Pagana TJ eds. Mosby’s Diagnostic and Laboratory Test Reference. 9th ed. St. Louis: Elsevier-Mosby; 2009
- Report from the National Diabetes Surveillance System (NDSS): Diabetes in Canada, 2009 Chronic Disease Surveillance Division. Centre for Chronic Disease Prevention and Control. Ottawa
- Rowland LP et al. (2010) Merritt’s Neurology (9th ed.) Philadelphia: Lippincoot Williams and Wilkins
- Skidmore-Roth L. ed. Mosby’s drug guide for nurses. 9th ed. St. Louis: Elsevier-Mosby; 2011
- Skidmore-Roth L, ed. Mosby’s nursing drug reference. 24th ed. St. Louis: Elsevier-Mosby; 2011
- http://www.diabetes.ca/files/for-professionals/CPGExecSummaryEssentials.pdf
Online Pharmacological Resources:
- e-CPS and e-Therapeutics
- Lexicomp
- RxList
- Epocrates
Journals/Clinical Trials:
- American Association of Clinical Endocrinologists Board of Directors, American College of Endocrinologists Board of Trustees. America Association of Clinical Endocrinologists/American College of Endocrinology statement on the use of hemoglobin A1c for the diagnosis of diabetes. Endocr Pract. 2010;16:155-156
- Cushman WC, Evans GW, Byington RP et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus.N Engl J Med 2010; 362:1575-1585
- Duckworth W, Abraira C, Moritz T, et al. Glucose Control and Vascular Complications in Veterans with Type 2 Diabetes N Engl J Med 2008; 358:2560-2572
- Espinola-Klein C, Rupprecht HJ, Bickel C et al. Different Calculations of Ankle-Brachial Index and Their Impact on Cardiovascular Risk Prediction. Circulation. 2008;118:961-967
- Gerstein HC, Miller ME, Byington RP et al. Effects of Intensive Glucose Lowering in Type 2 Diabetes N Engl J Med 2008; 358:2545-2559
- Ginsberg HN, Elam MB, Lovato LC et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010 29; 362:1563-74
- Grenon SM, Gagnon J, Hsiang Y. Ankle-Brachial Index for Assessment of Peripheral Arterial Disease N Engl. J. Med. 2009, 361 e40
- Harris, MI (ed). Diabetes in America, 2nd ed, National Institutes of Health Publication No. 95-1468, 1995
- Kahn B.B., Flier J.S. Obesity and insulin resistance J Clin Invest. 2000;106:473-481
- Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 2003; 289:76
- Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2006; 29:1963
- Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008 12; 358:2560-72
- Scognamiglio R, Negut C, Ramondo A, et al. Detection of coronary artery disease in asymptomatic patients with type 2 diabetes mellitus. J Am Coll Cardiol 2006; 47:65
- Trachtenbarg DE. Diabetic Ketoacidosis. Am Fam Physician. 2005 71:1705-1714
- WHO Consultation. Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus. WHO website. http://www.who.int/entity/diabetes/publications/report-hba1c_2011.pdf. Accessed June 14, 2011
