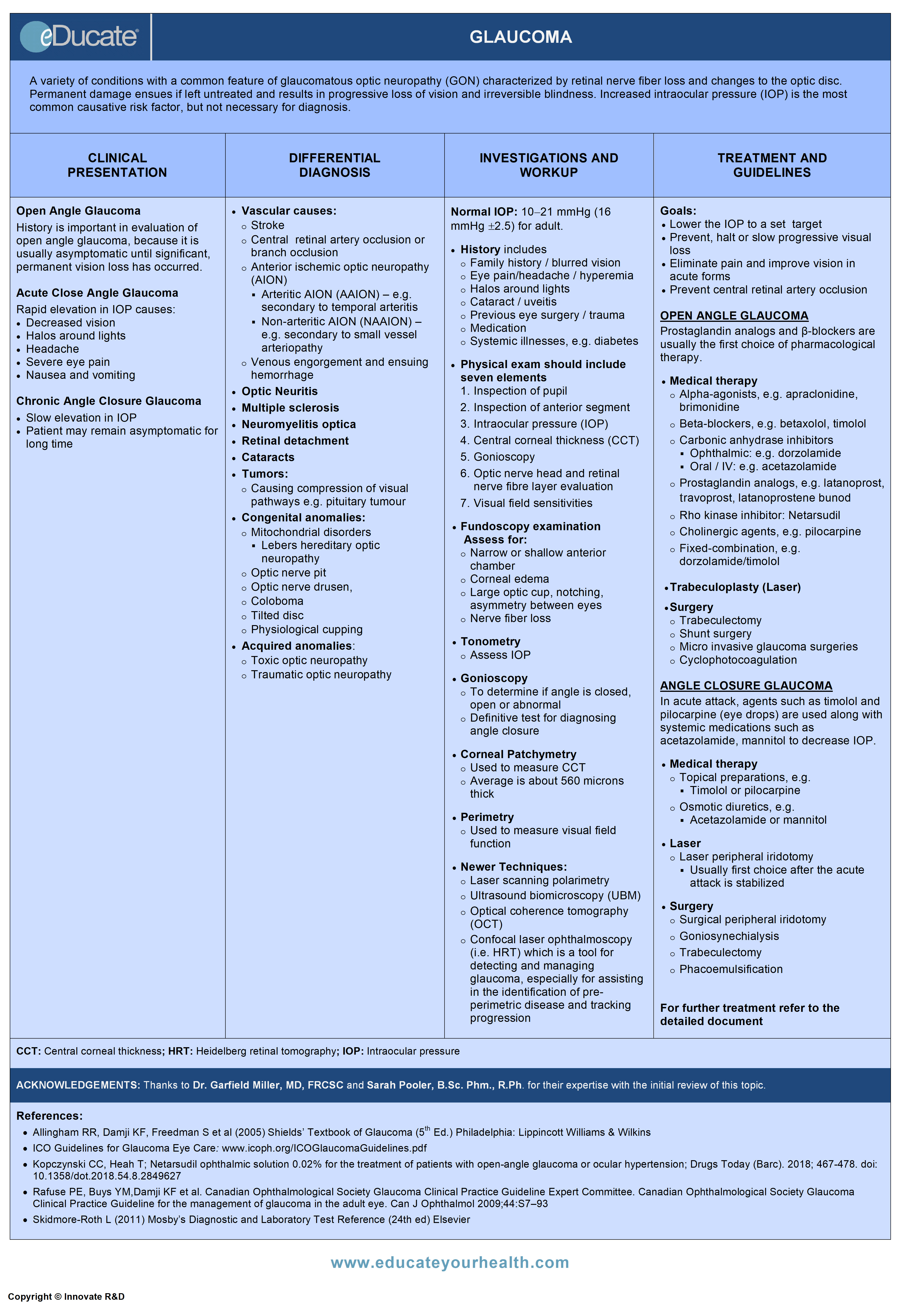
Glaucoma
ACKNOWLEDGEMENTS:
Thanks to Dr. Garfield Miller, MD, FRCSC, Assistant Professor of Ophthalmology, University of Ottawa, The Ottawa Eye Institute, The Ottawa Hospital, Ottawa, ON Canada, and Sarah Pooler, B.Sc. Phm., R.Ph., Drug Information Pharmacist, Drug Information and Research Centre, Ontario Pharmacists Association, Toronto, ON Canada for their expertise with the initial review of this topic.
Definition
A variety of conditions with a common feature of glaucomatous optic neuropathy (GON) characterized by loss of retinal nerve fibers and changes in the optic disc. Permanent damage ensues if left untreated and results in progressive loss of vision and irreversible blindness.
Increased intraocular pressure (IOP) is the most common causative risk factor but is not necessary for diagnosis.
Open and closed-angle glaucoma can be primary or secondary.
- Primary glaucoma: Refers to conditions in which there are no other associated ocular or systemic cause
- Secondary glaucoma: Refers to those conditions in which there is an associated ocular or systemic condition
- Congenital or developmental glaucoma: Represent only a small percentage of the overall cases of glaucoma and are usually identified prior to 3-5 years of age
Note: Open-angle glaucoma makes up ~90% of all primary glaucoma cases, while angle closure glaucoma makes up the other 10%, and its incidence increases with age.
1) Open-angle 2) Angel closure 3) Developmental
Etiology
Primary and secondary glaucoma may be associated with increased intraocular pressure (IOP).
Putative mechanisms of elevated IOP include:
Obstruction to aqueous outflow from:
- Closure of iridocorneal drainage due to apposition of the trabecular meshwork and iris root (closed-angle) or obstruction to outflow through the drainage pathways of an open angle
- Obstruction to the venous drainage of the eye
Other potential causes of increased IOP include:
- Trauma to outflow pathways
- Metabolic factors related to aqueous production
- Anatomic or physiologic features of the trabecular meshwork
- Mutations in the myocilin gene (MYOC) have been identified. Myocilin is produced in the ciliary body and in the trabecular meshwork
Closed-angle glaucoma develops if the high IOP causes glaucomatous damage to the optic disc.
Open-angle glaucoma occurs if damage to the disc is present in the face of an open angle.
Secondary causes that may lead to glaucoma include:
- Pseudo-exfoliation syndrome
- Pigment dispersion syndrome (pigmentary glaucoma)
- Lens-induced glaucoma
- Ocular inflammatory diseases
- Intraocular tumours
- Raised episcleral venous pressure (e.g. Sturge Weber syndrome, orbit tumor, carotid cavernous fistula)
- Neovascularization of trabecular meshwork (diabetes mellitus, vascular occlusions etc.)
Risk factors for glaucoma include
- Age
- Family history of glaucoma
- Elevated eye pressure (IOP)
- Nearsightedness (POAG)
- Farsightedness (angle closure)
- African, Hispanic or Asian ancestry
- Diabetes mellitus
- Previous eye injury
- Thin cornea
- Prolonged use of topical, periocular, inhaled, or systemic corticosteroids
Epidemiology
Canadian:
- Glaucoma is the leading cause of irreversible blindness
- Approximately affects 300,000 Canadians, ~80% are >40 years of age, while ~50% of these are unaware of there diagnosis
USA:
- Prevalence for primary open angle glaucoma (POAG)
- In the adult US population over age 40 is 1.86%
- In whites is 1.69%
- Age-adjusted prevalence in blacks is three times higher than in whites
Worldwide:
Approximately 3 million people are bilaterally blind from POAG.
Prevalence:
- Primary open angle glaucoma is 1.96%
- Angle-closure glaucoma is 0.69%
The incidence increases with age and is more prevalent among men.
Pathophysiology
Pathogenesis of open-angle glaucoma:
Although the exact mechanism is unknown, elevated IOP is often associated with the development of glaucoma.
The putative mechanism by which IOP initiates glaucomatous damage includes:
- Mechanical Hypothesis: Elevated IOP causes backward bowing of the lamina cribrosa, compressing the axons as they exit through the luminal pores, interfering with the axoplasmic flow of trophic factors, triggering cell death
- Vascular Hypothesis: Chronic hypoxia and ischemia leads to glaucomatous damage
Other potential mechanisms include:
- Excitotoxic injury from excessive retinal glutamate
- Deprivation of neuronal growth factors
- Peroxynitrite toxicity from increased nitric oxide synthetase activity
- Immune-mediated nerve damage, and oxidative stress
The above mentioned conditions lead to apoptotic cell death.
Pathogenesis of acute angle closure glaucoma (AACG):
Apposition of the iris to the trabecular meshwork at the angle of the anterior chamber, obstructs aqueous outflow from the eye leading to increased IOP.
In primary angle closure glaucoma, this apposition is due to a mechanism called Pupil Block.
- The normal flow of aqueous humour is from the posterior chamber, around the lens, through the pupil, into the anterior chamber, and out through the trabecular meshwork. In predisposed patients, the pupil margin touches the lens and can cause resistance to the normal flow through the pupil. Pressure builds in the posterior chamber causing the peripheral iris to bulge forward (iris bombe). If the peripheral iris covers the trabecular meshwork, outflow is obstructed
Note: Immediate treatment is required to prevent nerve damage and vision loss; symptoms are severe in sudden closure.
Pathogenesis of chronic angle closure glaucoma (CACG):
- May occur when portions of the anterior chamber angle are closed permanently by peripheral anterior synechiae (PAS)
Note: Eyes with progressive peripheral anterior synechiae (PAS) formation eventually may develop AACG when pupillary block results in closure of the remaining portions of the angle unaffected by PAS.
Combined mechanism glaucoma:
- Carry both components of open and closed angle glaucoma

Clinical Presentation
Open-Angle Glaucoma:
History is crucial in the evaluation of open-angle glaucoma as this condition is often asymptomatic until most of the vision is lost.
Acute Angle-Closure Glaucoma:
Triggered by rapid elevation in IOP resulting in:
- Decreased vision
- Halos around lights
- Headache
- Severe eye pain
- Nausea and vomiting
Chronic Angle-Closure Glaucoma:
- Patients remain asymptomatic for a long time due to slow elevation in IOP
Differential Diagnosis
Other optic neuropathies
a) Vascular Causes:
- Stroke
- Central retinal artery occlusion or branch occlusion
- Anterior ischemic optic neuropathy (AION)
- Arteritic AION (AAION) – e.g. secondary to temporal arteritis
- Non-arteritic AION (NAAION) – e.g. secondary to small vessel arteriopathy
- Venous engorgement and ensuing hemorrhage
b) Optic neuritis
c) Multiple sclerosis/neuromyelitis optica
d) Retinal detachment
e) Cataracts
f) Tumors:
- Causing compression of visual pathways e.g. pituitary tumour
g) Congenital anomalies:
- Mitochondrial disorders
- Lebers hereditary optic neuropathy
- Optic nerve pit
- Optic nerve drusen
- Coloboma
- Tilted disc
- Physiological cupping
h) Acquired anomalies:
- Toxic optic neuropathy
- Traumatic optic neuropathy
Investigation and Workup
History should include:
- Birth and developmental history
- Family history
- Episodes of eye pain/headache/nausea/blurred vision (acute glaucoma)
- Halos around lights (acute glaucoma)
- Cataract
- Uveitis
- Previous eye surgery/trauma
- Systemic illnesses
- Diabetes
- Hypertension
- Cardiovascular disease
- Migraines
- Raynaud’s disease
- Use of medicines affecting intraocular pressure (steroids-systemic, ocular, inhaled)
Physical examination should include assessment of:
- Pupil
- Anterior segment
- Central corneal thickness (CCT)
- Gonioscopy
- Optic nerve head and retinal nerve layer evaluation
- Visual field sensitivities
- Intraocular pressure (IOP)
Normal intraocular pressure
- Adult: 10-21 mmHg (16 mmHg ±2.5); tends to increase with age
- Children: Increases ~1 mmHg every 2 years till 12 years
- At birth 6-8 mmHg
- At 12 years 12 ±3 mmHg
- Diurnal variation: IOP often follows circadian rhythm
- Maximum: Between 8 a.m. and 11 a.m.
- Minimum: Between midnight and 2 a.m.
- Variation: Between 3 and 5 mmHg
Tonometry: To measure IOP in millimeters of mercury(mmHg)
- Multiple readings from hour to hour are taken
- IOP in both eyes are measured
- Comparison with previous readings are done
- Difference of 3 mmHg in both eyes is an indication for further investigation for glaucoma
- Factors that need to be taken into account when measuring IOP include:
- Age, gender, race
- Refraction
- Posture, holding breath, tight collar/tie
- Exercise
- Blood pressure
- Corneal thickness
- Ocular movement
- Variety of drugs
Corneal Pachymetry:
- Used to check central corneal thickness(CCT)
- Independent risk factor for development of glaucoma
- Average cornea is about 560 microns thick
- Generally, patients with thin cornea (less than 555 µm) show artificially low IOP readings
- Patients with thicker CCT (589 microns or more) show a higher reading of IOP than actually exists
Gonioscopy:
- Used to determine if the angle is open, closed or abnormal
Fundoscopy:
- Direct ophthalmoscopy: Produces an upright, or unreversed, image of approximately 15 times magnification
- Indirect ophthalmoscopy: Produces an inverted, or reversed, direct image of 2 to 5 times magnification
- Findings:
- Evidence of glaucomatous optic nerve damage
- Thinning or notching of the disc rim
- Retinal nerve fiber layer defects
Slit lamp examination:
Focus on cornea, anterior chamber, lens, optic nerve fibers, and fundus.
- Narrow-angle or shallow anterior chamber
- Von Herrick’s sign: If peripheral anterior chamber depth is ¼ or less of the thickness of peripheral cornea, angle-closure possible
- Corneal edema
- Large optic cup
- Cup to Disc Ratio: Disc >0.5 or asymmetry between eyes of >0.1 are risk factors
- Narrowing, notching, or pallor of the neuroretinal rim
- Splinter hemorrhage at disk
- Nerve fiber layer defects
- Beta peri-papillary atrophy (pigment changes directly adjacent to optic disc)
Perimetry (visual fields):
- It is the systematic measurement of visual field function, that is central and peripheral (side) vision
- It is a crucial component of glaucoma diagnosis, and more importantly, for follow up
Laboratory:
Routine labs are not routinely done, unless considering normal tension glaucoma or other optic neuropathies.
- CBC, ESR, electrolytes
- Serology for syphilis
Imaging:
Methods of measuring the optic disc and the nerve fiber layer:
- Laser scanning polarimetry (e.g. GDx Nerve Fiber Analysis System)
- Measures the thickness of the nerve fiber layer
- Confocal laser ophthalmoscopy – (e.g. Heidelberg Retinal Tomography or HRT)
- Scans layers of the retina to make quantitative measurements of the surface features of the optic nerve head and fundus
- Optical coherence tomography (OCT)
- OCT can create a contour map of the optic nerve, optic cup and measure the retinal nerve fiber thickness
- New anterior segment OCT machines are helpful in the evaluation of angle closure
- Stereo photographs
- Used to determine the three-dimensional characteristics of the optic nerve head, and for following glaucomatous change of the optic nerve head over time
- Ultrasound biomicroscopy (UBM) – (High-resolution ultrasound technique)
- Helpful to obtain a better view of the angle, iris, and ciliary body structures to rule out anatomical pathology and secondary causes of elevated IOP
Treatment
- Stop progression and maintain quality-of-life
- Lower IOP to a desired pressure; if causing damage
- Identify and treat underlying causes
OPEN-ANGLE GLAUCOMA
Indication for treatment:
- Patients with high IOP may or may not require treatment; the latter is based on evidence of ongoing damage, rather than a specific IOP
- Some patients with normal IOP may require treatment, if there is an evidence of ongoing injury and visual field loss
- Target pressures should be set and achieved, but treatment is generally escalated until there is no more evidence of progression
- Choice of therapeutic agents depends on patients disease status, target ocular pressure, medical history, pharmacoeconomics and psychological factors
Medical therapy:
Alpha-agonists: Suppress aqueous inflow and include
- Apraclonidine
- Brimonidine
Red-eye and ocular irritation is often associated with the use; caution required in pregnancy, contraindicated if patient is taking monoamine oxidase inhibitors(MAO).
Beta-blockers: Suppress aqueous inflow and include
- Betaxolol
- Carteolol (only available in the US)
- Levobunolol
- Metipranolol (only available in the US)
- Timolol
Carbonic anhydrase inhibitors: Suppress aqueous inflow and include
- Dorzolamide (topical)
- Brinzolamide (topical)
- Acetazolamide (oral)
- Methazolamide (oral)
Prescribed when topical treatment is not effective
May cause hypokalemia if sodium and potassium levels are depressed as in kidney or liver disease caution is advised.
Prostaglandin analogs: Increase outflow and include
- Latanoprost
- Travoprost
- Bimatoprost
- Tafluprost
- Latanoprostene bunod
Discoloration of iris and foreign body sensation is a common complaint; caution advised on use during pregnancy.
Rho kinase inhibitor: suppressing the rho kinase enzymes responsible for fluid increase
- Netarsudil
Conjunctival hyperemia is a common complaint.
Cholinergic agents: Facilitates aqueous outflow
- Pilocarpine
- Carbachol
Headaches and dim vision is a common complaint.
Interventional Therapy:
Laser therapy:
- Laser trabeculoplasty
- Increases aqueous outflow
- The effect typically is not permanent
- Laser can be repeated (especially Selective Laser Trabeculoplasty-SLT)
- Cyclophotocoagulation
- Diode laser applied directly through sclera targeting ciliary body
- Decreases aqueous production
- Usually reserved for eyes with poor visual potential
Surgery:
- Trabeculectomy: Creation of a filtration bleb to allow egress of aqueous humor from the eye. An artificial fistula is made between the anterior chamber and a formed space underneath the conjunctiva
- Aqueous shunt: Small silicone tube implanted in the anterior chamber that leads to a baseplate placed underneath the conjunctiva. Shunts are typically used in patients who have failed conventional surgery or have an underlying diagnosis that increases the risk of surgical failure
- Endocyclophotocoagulation: Endoscopic visualization and application of laser to the ciliary processes (where aqueous humour is produced.) Often done at the time of cataract surgery. Moderate IOP lowering
MICRO invasive glaucoma surgeries (minimally invasive glaucoma surgeries):
Are newer surgical procedures, which may carry less risk and morbidity than traditional procedures. In general, these interventions do not lower IOP when compared with others, and often used for less advanced cases.
- Schlemm’s Canal procedures
- iStent trabecular micro-bypass – smallest implant in human body. L-shaped stent placed through trabecular meshwork into Schlemm’s canal. Usually done in conjunction with cataract surgery
- Trabectome – Electrocautery used to ablate trabecular meshwork, opening up Schlemm’s canal. Often done in conjunction with cataract surgery
- Canaloplasty – Schlemm’s canal is approached externally with meticulous dissection. A fiber optic is used to canulate the canal 360 degrees, then a suture is left in the canal and tied with tension to expand it. The anterior chamber is not entered with this technique
- Suprachoroidal procedures – Various procedures shunting aqueous humour from the anterior chamber into the suprachoroidal space. This space is found between the choroid and the sclera. These procedures have not yet achieved widespread use
ANGLE-CLOSURE GLAUCOMA
Goal of treatment:
- Prevention and reversal of angle closure
- Control of intraocular pressure
Indications for treatment:
- Patients with signs or symptoms suggesting possible acute angle closure should be referred for emergent assessment and treatment to prevent permanent damage
Treatment options:
In acute emergencies, treatment usually involves, combination of both topical and oral agents.
Topical preparations
- All classes of pressure lowering drops should be used
- Topical steroid
Systemic therapy
- Oral acetazolamide- 250 mg two tablets stat. The eye pressure should be checked 30 to 60 minutes after giving oral acetazolamide. Used mainly to temporize until definitive treatment (surgery) can be arranged or underlying cause removed (e.g. steroid effect)
- IV mannitol
- Oral glycerol or isosorbide
Laser peripheral iridotomy
- Once the episode is controlled the treatment of choice is a laser peripheral iridotomy
- This procedure creates a tiny hole in the peripheral iris allowing drainage of aqueous humor and equalizing the pressure between the anterior and posterior chambers, thus relieving pupil block
- Reassess the IOP 30 to 120 minutes after the iridotomy
- If it is not possible to perform laser iridotomies, surgical peripheral iridectomy may be necessary
- Laser peripheral iridotomy is also indicated in the fellow eye as prophylaxis. It is recommended that all patients with narrow angles, symptomatic or not, should have prophylactic iridotomies performed
Complications of laser peripheral iridotomy
- Increased IOP
- Inflammation
- Laser burns to the cornea, lens, or retina
- Glare or “white line” in vision
- Development of cataract
- Need for repeat treatment if spontaneous closure occurs
Other surgical procedures
- Goniosynechialysis: Mechanical lysis of peripheral anterior synechiae to restore drainage function
- Phacoemulsification: Removing the lens that is crowding the angle and replacing it with a flat intraocular lens implant
[/cq_vc_tab_item][cq_vc_tab_item tabtitle=”Medication Dose”]MEDICATIONS:
- Apraclonidine
- Brimonidine
Mechanisms:
- It is selective alpha2-receptors agonist with some binding to alpha1-receptors
- Decreases aqueous humor formation
- Produces local vasoconstriction and reduction in blood flow in the eye
Brimonidine (additional)
- Increased uveoscleral outflow
Dose:
Apraclonidine
Glaucoma/Tonometry/Gonioscopy
- 1-2 gtt 0.5% solution in the affected eye TID
Postsurgical intraocular pressure elevation
- 1 gtt 1% solution in the operative eye 1 hour before surgery; Then 1 gtt immediately upon completion of a procedure
Brimonidine
Glaucoma/Ocular hypertension
- 0.2% (1 gtt) in affected eye twice daily
- Timolol
- Betaxolol
- Levobunolol
Mechanisms:
- Reduces the production of aqueous humor
Timolol/Levobunolol
- Blocks both beta1– and beta2-adrenergic receptors
Betaxolol (additional)
- Selectively blocks beta1-receptors antagonist, with little or no effect on beta2-receptors
Dose:
Timolol
Glaucoma/Elevated IOP
- Solution: 1 gtt of 0.25% solution BID into affected eye(s); Max. 1 gtt of 0.5% solution BID
- Note: Decrease to 1 gtt one daily if controlled.
- Gel-forming solution: 1 gtt of either 0.25% or 0.5% once daily into affected eye(s)
Betaxolol, Levobunolol
Open-angle glaucoma/Elevated IOP
- 0.5% solution: 1-2 gtt BID into affected eye(s)
- 0.25% suspension: 1 gtt BID into affected eye(s)
Carbonic anhydrase inhibitors (ophthalmic)
- Dorzolamide
- Brinzolamide
Mechanisms:
- Reversible inhibition of the carbonic anhydrase enzyme produces
- Reduction of hydrogen ion secretion at renal tubule
- Increased renal excretion of sodium, potassium, bicarbonate, and water to decrease production of aqueous humor
- This results in decreased production of aqueous humor
Dose:
Dorzolamide
Open-angle glaucoma / Ocular hypertension
- 1 gtt of 2% solution TID in the affected eye(s)
- Dose in Renal Impairment: Avoid use if CrCl <30
Brinzolamide
Open-angle glaucoma / Ocular hypertension
- 1 gtt 1% suspension TID in affected eye(s)
Carbonic anhydrase inhibitors (Oral/IV)
- Acetazolamide
- Methazolamide
Mechanisms:
- Reversible inhibition of the enzyme carbonic anhydrase resulting in
- Reduction of hydrogen ion secretion at renal tubule
- Self-limiting urinary excretion of sodium, potassium, bicarbonate, and water
- Alkaline diuresis prevents precipitation of uric acid or cystine in the urinary tract
- CNS inhibition of carbonic anhydrase and resultant diuresis may decrease abnormal discharge from CNS neurons
- Methazolamide is not considered an effective anticonvulsant
Dose:
Acetazolamide
Acute angle-closure glaucoma/Secondary glaucoma
- 250 mg PO/IV every 4 hrs
- In acute cases: initial 500 mg IV followed 125-250 mg every 4 hrs is preferable
- Short-term therapy with 250 mg twice daily, some adults may respond to this as well
Chronic simple (open-angle)
- 250 mg PO/IV once daily or in 2-4 divided doses up to 1g/day. Or 500 mg PO BID of Extended-release capsules
Dose in renal impairment:
- If CrCl 10-50 mL/min give only 12 hourly
- CrCl <10 avoid use
Methazolamide
- 50-100 mg PO BID or TID
Prostaglandin agonist, (ophthalmic)
- Latanoprost
- Travoprost
- Bimatoprost
- Tafluprost
- Latanoprostene bunod
Mechanisms:
- Exact mechanism is unknown
- Possibly it decreases the IOP by increasing uveoscleral outflow of aqueous humor
Dose: In glaucoma and ocular hypertension
Latanoprost
- 1 gtt at night in the affected eye(s)
Travoprost
- 1 gtt at night in the affected eye(s)
Bimatoprost
- 1 gtt at night in the affected eye(s), do not exceed once daily dosing
Tafluprost
- 1 gtt at night in the affected eye(s)
Latanoprostene bunod
- 1 gtt at night in the affected eye(s), do not exceed once daily dosing
Rho kinase inhibitor:
- Netarsudil
Mechanisms:
- Exact mechanism is unknown
- Suppress the rho kinase enzymes responsible for fluid increase
- May reduce IOP by increasing the outflow of aqueous humor through the trabecular meshwork route
Dose: In open-angle glaucoma
Netarsudil
- 1 gtt 0.02% solution at night in the affected eye(s)
Cholinergic agents, (ophthalmic)
- Pilocarpine
- Carbachol
Mechanisms:
Directly stimulates cholinergic receptors in the eye causing
- Constriction of the pupil by contracting the iris sphincter
- Loss of accommodation by contracting the ciliary muscle
- Lower the intraocular pressure and facilitates aqueous humor outflow
Dose:
Pilocarpine
Open-angle glaucoma
- 1-2 gtt of 1-4% solution 6 times a day
- 0.5 inch strip of 4% gel into lower conjunctival sac at bedtime
Acute angle-closure glaucoma
- 1 gtt of 2% solution in the affected eye every 5-10 mins for 3-6 doses, followed by 1 drop every 1-3 hours until pressure is controlled
Carbachol
Open-angle glaucoma
- 1-2 gtt of 1.5-3% solution 3 times a day
- Mannitol
Mechanisms:
- Increases the osmotic pressure of glomerular filtrate, which inhibits tubular reabsorption of water and solutes and increases urinary output
- It increases the excretion of Sodium, Potassium, Chloride, Calcium, Phosphorus, Lithium, Magnesium, Urea, and Uric acid
Dose:
Mannitol
Reduction of intraocular pressure
- 1.5-2 g/kg IV as a 15-25% solution over 30-60 minutes
- Glycerol
Mechanisms:
- Osmotic dehydrating agent increases osmotic pressure
- It draws fluid into lumen and also from the extravascular spaces into intravascular compartment
- It also irritates luminal mucosa and increases peristalsis
Dose:
Glycerol
Reduction of intraocular pressure
- 1-1.8 g/kg PO1-11/2 hours preoperatively; may administer additional doses at 5 hour intervals
Fixed-combination preparations, (ophthalmic)
- Carbonic anhydrase inhibitors/Beta-blockers, (ophthalmic)
- Dorzolamide/Timolol
- Brinzolamide/Timolol
- Prostaglandin agonist/Beta-blockers, (ophthalmic)
- Latanoprost/Timolol
- Travoprost/Timolol
- Alpha-2 agonist/Beta-blockers, (ophthalmic)
- Brimonidine/Timolol
Dose:
- Dorzolamide/Timolol
- 1 gtt BID into affected eye(s)
- Brinzolamide/Timolol
- 1 gtt BID into affected eye(s)
- Latanoprost/Timolol
- 1 gtt daily into affected eye(s)
- Travoprost/Timolol
- 1 gtt once every morning into affected eye(s)
- Brimonidine/Timolol
- 1 gtt BID into affected eye(s)
[/cq_vc_tab_item][/cq_vc_tabs]
Pipeline Agents
NOTHING NEW AT THE MOMENT.
Physician Resources
1. Tips for Patient Care
- Early detection and treatment of glaucoma is always important to control the disease progress
- Most primary angle closure glaucoma’s are bilateral
- Ensure patient is well informed about the disease and its treatment
- Encourage compliance – this is one of the most significant barriers to control of the disease
Medications:
- Inform patients of the proper administration techniques as follows
- Tip of the eye drop bottle should not touch the eyelashes
- Administration should be conducted one drop at a time
- After administration, gently close the eye and compress the lacrimal sac
- Allow at least 5 minutes interval between multiple eyes drops use
- Drugs with Anticholinergic activity can precipitate angle-closure glaucoma
- Compliance issues must be verified before changing medications
- Appropriate therapeutic modality must be selected based on the individual patient and the disease stage and type
- In Angle-closure glaucoma, risk of permanent vision loss is minimized if the patient is treated promptly
Social and stress factors:
- Inadequate or incomplete information about the disease and its treatment is one of the most distressing factors for the patient and family
- Optic nerve damage and visual field damage caused by glaucoma is essentially progressive and irreversible, which are typical stressors
- Provide social and psychological support, and discuss in detail
Physical activity:
- Maintain regular activity but avoid overwork and fatigue
- Advise patient to rest during periods of acute relapse
Expected outcome
- Optic nerve damage and visual field damage caused by glaucoma is essentially progressive and irreversible
- Once visual function has been lost in glaucoma, there is no way to regain it
- Risk of permanent vision loss is minimized if the patient is treated promptly
- Outcome depends on risk factors for progression and includes:
- Pre-diagnosis high mean intraocular pressure
- Higher anticardiolipin antibodies ( ACA) levels
- Older age at the time of diagnosis and female gender
- Time to treatment
- Underlying eye disease
- Ethnicity
Ref: Canadian Ophthalmological Society Glaucoma Clinical Practice Guideline Expert Committee. Canadian Ophthalmological Society evidence-based clinical practice guidelines for the management of glaucoma in the adult eye. Can J Ophthalmol 2009; 44:S7-S93.
2. Scales and Tables
References
Core Resources:
- Allingham RR, Damji KF, Freedman S, et al (2005) Shields’ Textbook of Glaucoma (5thed.) Philadelphia: Lippincott Williams & Wilkins
- Compendium of Pharmaceuticals and Specialties (CPS). Canadian Pharmacist Association. Toronto: Webcom Inc. 2012
- Day RA, Paul P, Williams B, et al (eds). Brunner & Suddarth’s Textbook of Canadian Medical-Surgical Nursing. 2nd ed. Philadelphia: Lippincott Williams and Wilkins; 2010
- European Glaucoma Society Terminology and Guidelines for Glaucoma, 4th Edition – Chapter 2: Classification and terminology Supported by the EGS Foundation: Part 1: Foreword; Introduction; Glossary; Chapter 2 Classification and Terminology. Br J Ophthalmol. 2017;101(5):73‐127. doi:10.1136/bjophthalmol-2016-EGSguideline.002
- Gray J, ed. Therapeutic Choices. Canadian Pharmacists Association. 6th ed. Toronto: Webcom Inc. 2011
- Hoy SM. Latanoprostene Bunod Ophthalmic Solution 0.024%: A Review in Open-Angle Glaucoma and Ocular Hypertension [published correction appears in Drugs. 2018;78:857]. Drugs. 2018;78(7):773‐780. doi:10.1007/s40265-018-0914-6
- Kopczynski CC, Heah T; Netarsudil ophthalmic solution 0.02% for the treatment of patients with open-angle glaucoma or ocular hypertension; drugs Today (Barc). 2018;54(8):467-478. doi: 10.1358/dot.2018.54.8.2849627
- Leary, KA, Lin, K‐T, Steibel, JP, Harman, CD, Komáromy, AM. Safety and efficacy of topically administered netarsudil (Rhopressa™) in normal and glaucomatous dogs with ADAMTS10‐open‐angle glaucoma (ADAMTS10‐OAG). Vet Ophthalmol. 2019; 00: 1– 12. https://doi.org/10.1111/vop.12734
- Liu Y, Mao W. Tafluprost once daily for treatment of elevated intraocular pressure in patients with open-angle glaucoma. Clin Ophthalmol. 2013;7:7‐14. doi:10.2147/OPTH.S30951
- Longo D, Fauci A, Kasper D, et al (eds). Harrison’s Principles of Internal Medicine. 18thed. New York: McGraw-Hill; 2011
- McPhee SJ, Papadakis MA, eds. Current Medical Diagnosis & Treatment. 49th ed. New York: McGraw-Hill; 2010
- Pagana KD, Pagana TJ eds. Mosby’s Diagnostic and Laboratory Test Reference. 9th ed. St. Louis: Elsevier-Mosby; 2009
- Rafuse PE, Buys YM,Damji KF et al. Canadian Ophthalmological Society Glaucoma Clinical Practice Guideline Expert Committee. Canadian Ophthalmological Society Glaucoma Clinical Practice Guideline for the management of glaucoma in the adult eye. Can J Ophthalmol 2009;44:S7-93
- Skidmore-Roth L. ed. Mosby’s drug guide for nurses. 9th ed. St. Louis: Elsevier-Mosby; 2011
- Skidmore-Roth L, ed. Mosby’s nursing drug reference. 24th ed. St. Louis: Elsevier-Mosby; 2011
- Yanoff M and Duker J S (2009) Yanoff and Duker: Ophthalmology (3rd ed.) Edinburgh: Elsevier
Online Pharmacological Resources:
- e-therapeutics
- Lexicomp
- RxList
- Epocrates Online
Journals/Clinical Trials:
- Chauhan BC, Mikelberg FS, Balaszi AG, et al; Canadian Glaucoma Study Group. Canadian Glaucoma Study: 2. Risk factors for the progression of open-angle glaucoma Arch Ophthalmol. 2008 Aug; 126(8):1030-6
- Francis BA, Singh K, Lin SC, et al. Novelglaucoma procedures: a report by the American Academy of Ophthalmology. Ophthalmology 2011; 118: 1466-80
- Heijl A, Peters D, Leske MC, et al. Am J Ophthalmol.2011; 152: 842-8
- Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002; 120:701-13
- Van Buskirk EM, Weinreb RN, Berry DP, et al. Betaxolol in patients with glaucoma and asthma. Am J Ophthalmol. 1986 May 15;101:531-4
