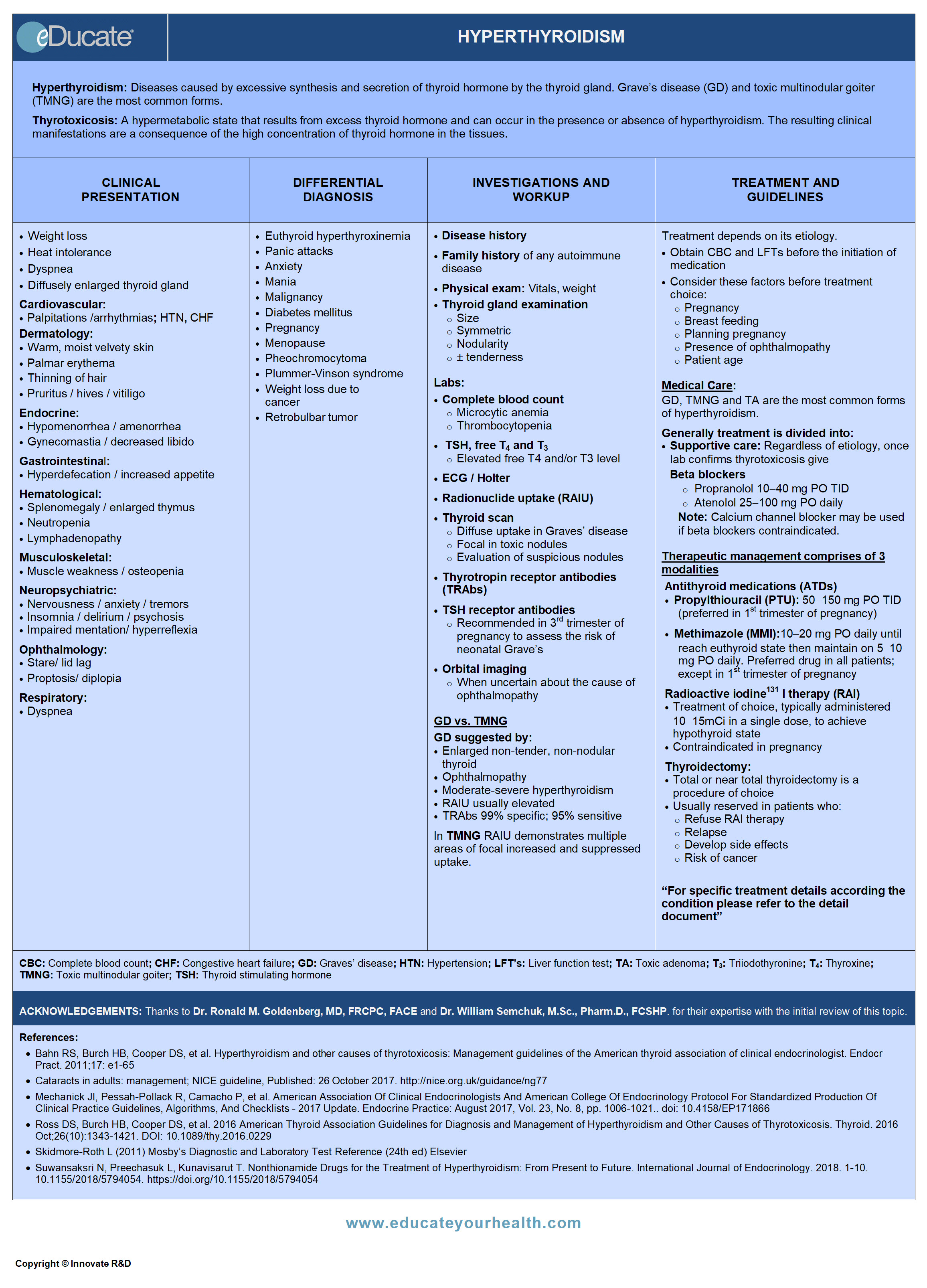
Hyperthyroidism
ACKNOWLEDGEMENTS:
Thanks to Dr. Ronald M. Goldenberg, MD, FRCPC, FACE, Staff Endocrinologist, North York General Hospital, ON Canada; Endocrinologist & Medical Co-Director, LMC Endocrinology Centres, Toronto, ON Canada, and Dr. William Semchuk, M.Sc., Pharm.D., FCSHP., Manager, Clinical Pharmacy Services, Regina Qu’Appelle Health Region 14, Regina, SK Canada for their expertise with the initial review of this topic.
[pdf-embedder url=”https://www.educateyourhealth.com/wp-content/uploads/2019/03/Hyperthyroidism-Brochure.pdf” width=”750″]
Definition
Hyperthyroidism:
- A group of diseases caused by excessive synthesis and secretion of thyroid hormone by the thyroid gland
Thyrotoxicosis:
- A hypermetabolic state that results from excess thyroid hormone and can occur in the presence or absence of hyperthyroidism. The resulting clinical manifestations are a consequence of the high concentration of thyroid hormone in the tissues
Thyrotoxic crisis/ thyroid storm:
- Rare but life-threatening exacerbation of hyperthyroidism, usually precipitated by acute illness or surgery
Etiology
The most common cause of thyrotoxicosis is Graves’ disease in which thyrotropin receptor antibodies (TRAbs) binds to and stimulates thyroid stimulating hormone receptors (TSHR) on the thyroid cells and results in increased production of T3 and T4.
The following table document causes of thyrotoxicosis and hyperthyroidism.
Epidemiology
- Prevalence of hyperthyroidism:
- Approximately 1.3% and increasing
- Age predominance:
- Graves’ disease (diffuse toxic goiter): 20-40 years
- Toxic multinodular goiter (TMNG) is the primary cause of hyperthyroidism in elderly patients
- Incidence:
- Increases with age; highest being in the white population and in iodine deficient areas
- Among women = 0.38/1000/year
- Common in smokers
Ref: Franklyn JA, Boelaert K. Thyrotoxicosis. Lancet 2012; 379: 1155-66
Pathophysiology

- Thyroxine (T4) is a prohormone and is present in higher concentrations than T3
- T3 is biologically active through interaction with specific nuclear receptors that are present in nearly all tissue
- T3 regulates energy production and metabolic rates; it has profound effects on cardiac, hepatic and neuromuscular functions, as well on fetal and postnatal growth and development
- Thyroid hormone concentration is regulated by negative feedback of circulating free hormone primarily on the anterior pituitary gland and to a lesser extent on the hypothalamus
General pathological mechanisms leading to thyrotoxicosis and hyperthyroidism:
- Inappropriate thyroid stimulation by trophic factors
- Continuous activation of thyroid hormone synthesis and secretion, resulting in independent release of increased thyroid hormone
- Preformed thyroid hormone is released in excessive amounts; due to abnormal autoimmune, infectious, chemical, or mechanical insults
- Extrathyroidal source of thyroid hormone; which may be endogenous (e.g. struma ovarii, metastatic thyroid cancer) or exogenous (e.g. factitious thyrotoxicosis)
Specific pathological mechanisms; involved in conditions associated with hyperthyroidism
Clinical Presentation
Elderly may have few symptoms (apathetic hyperthyroidism) or only present with weight loss or atrial fibrillation.
Heat Intolerance and weight loss are common.

Differential Diagnosis
See “Etiology” as well.
- Euthyroid hyperthyroxinemia
- Panic attacks
- Anxiety
- Mania
- Malignancy
- Diabetes mellitus
- Pregnancy
- Menopause
- Pheochromocytoma
- Plummer-Vinson syndrome
- Weight loss due to cancer
- Retrobulbar tumor
Investigation and Workup
History:
Detailed history includes:
- Palpitation
- Anxiety/ nervousness
- Increased perspiration
- Abrupt mood changes
- Heat/cold intolerance
- Menstrual irregularities in females
- Weight changes
- Family history of any autoimmune disease
Obtain vital signs, body weight, and perform local thyroid examination.
– Thyroid (anterior neck) examination:
- Size/ symmetry
- Nodularity
- Presence or absence of tenderness
– Ophthalmologic examination:
Approximately 50% of patients with hyperthyroidism may have associated mild thyroid ophthalmopathy, and may present with:
- Conjunctival edema
- Poor lid closure
- Extraocular muscle dysfunction causing diplopia
- Proptosis
– Dermatologic examination:
- Non-pitting edema
- Erythema
- Thickening of skin without pain or itching
– Diagnostic approach in hyperthyroidism:
Laboratory studies:
Patients with low serum TSH and high free T4 and/or T3 concentrations are hyperthyroid.
– Complete blood count
– TSH level
- If TSH normal – unlikely to be hyperthyroidism
- Suppressed in hyperthyroidism
- Hyperthyroidism with TSH less than 0.3 mU/L (or undetectable) with normal free T4 estimates and normal total T3 or free T3 estimates, usually indicative of subclinical disease
– Serum free T4 and T3
- May be used to confirm diagnosis
- Elevated free T4 and/or T3 level
- In Graves’s (or nodular) associated hyperthyroidism T3 > T4
– ECG/Holter monitor
Specific to Grave’s Disease (GD)
– TSH receptor antibodies: Results are valuable during
- Pregnancy
- After recent iodine load
GD is an autoimmune disorder in which thyrotropin receptor antibodies (TRAbs) stimulate the TSH receptor, increasing thyroid hormone production.
– Orbital imaging
- Evaluate other causes of ophthalmopathy
Imaging:
– Radionuclide uptake:
- Can help distinguish hyperthyroidism from other causes of thyrotoxicosis)
– Thyroid scan I-123 or Technetium scintigraphy:
May help determine the etiology of hyperthyroidism.
- Diffuse uptake in Grave’s disease
- Focal in toxic nodules
- Evaluation of suspicious nodules
– Ultrasound with fine needle aspiration of suspicious nodules
– Pituitary MRI: Rule out pituitary adenoma
Treatment
- Assessment of disease severity and underlying cause as determined by history, physical and investigations
- Factors that are important to consider in the choice of treatment:
- Pregnancy
- Breastfeeding
- Planning pregnancy
- Presence of ophthalmopathy
- Patient’s age
- Obtain CBC and LFTs before the initiation of antithyroid medication.
Supportive care:
Regardless of etiology, beta blockers may be started as soon as in laboratory confirmed thyrotoxicosis, except in patients with significant history of asthma; where it is contraindicated.
Note: Calcium channel blockers (verapamil and diltiazem) may be used for rate control in patients intolerant to beta blockers, or in whom it is contraindicated.
Management of overt hyperthyroidism due to Graves’ disease:
Patients should be treated with any of the following modalities i.e.
- 131I therapy ( radioactive iodine)
- Antithyroid drugs (ATDs)
- Surgery
Typically in North America, physicians prefer radioactive iodine, while in Europe and Japan ATD’s/or surgery is preferred. However, the long-term quality of life following any one of the treatment modality is the same.

- 131I therapy in the management of Graves’ disease is commenced as shown in the table:
- Antithyroid drugs in the management of Graves’ disease as shown in the table:
- Thyroidectomy in the management of Graves’ disease as shown in the table:
Management of thyroid nodule in patients with Graves’ disease:
Thyroid nodule larger than 1-1.5 cm should always be evaluated prior to radioactive iodine therapy in GD. The occurrence of thyroid cancer is approximately ≤2% in GD.
Following steps are recommended on discovery of a thyroid nodule:
- Any nonfunctioning or hypoactive nodule should be considered for fine needle aspiration
- If pathology is suspicious or confirms malignancy, surgery is advised after normalization of thyroid function
Management of thyroid storm associated complication:
Thyroid storm is a life-threatening disorder characterized by involving multiple systems with high mortality if not treated immediately and aggressively.
Criteria for thyroid storm has been defined by Burch- Wartofsky score; is solely based on clinical and physical assessment, which covers the following factors:
- Body temperature
- Central nervous effects
- Hepatic and gastrointestinal symptoms
- Cardiovascular dysfunction
Treatment comprises of:
- Provide immediate IV fluids, oxygen, ventilatory support, and ICU monitoring if needed
- Aggressive cooling with cooling blankets; followed by acetaminophen 15 mg/kg every 4 hrs, orally or rectally is administered in hyperthermia
- Administer antithyroid drugs to block the synthesis of new hormone; methimazole (MMI) 60-80 mg/day, or propylthiouracil (PTU) 500-1000 mg loading dose, then 250 mg every 4 hourly
- Beta-blockers (propranolol 60-80 mg every 4 hrs); it blocks T4 to T3 conversion and also used in patients with congestive cardiac failure (CCF)
- Iodine (SSKI) 5 drops (0.25 ml or 250 mg) PO every 6 hrs, to block thyroid hormone release and new hormone synthesis
- To further block the conversion of T4-T3, and avoid adrenal insufficiency; administer hydrocortisone 300 mg IV loading dose followed by 100 mg every 8 hrs
Management of overt hyperthyroidism due to TMNG or TA:
Treatment is focused to achieve rapid and sustained euthyroid state, there are two main treatment modalities recommended.
- 131I therapy
- Thyroidectomy
Note: Sometimes lifelong ATDs are the best choice for elderly patients who are in nursing homes, have increased surgical risk and are with limited life expectancy. The treatment requires frequent monitoring (every 3 months).
- Factors and contraindications influencing the decision to choose particular modality:
- 131I therapy in the management of overt hyperthyroidism due to TMNG or TA is commenced as shown in the table:
- Thyroidectomy to manage TMNG or TA as shown in the table:
Management of subclinical hyperthyroidism:
Subclinical hyperthyroidism is defined by low TSH less than 0.3 mU/L (or undetectable) with normal free T4 estimates and normal total T3 or free T3 estimates.
- Usually, an incidental finding during a routine physical or sometimes signs and symptoms suggest the possibility of hyperthyroidism to the physician
- Once the results are confirmed by repeating the labs, determine the cause of low TSH by excluding other causes of decrease TSH levels such as:
- Pituitary and hypothalamic disease
- Euthyroid sick sinus syndrome
- Drugs e.g. dobutamine, dopamine and steroids
- Pregnancy: First trimester
- Factitial TSH suppression e.g. excessive thyroid hormone ingestion
- In elderly TMNG is probably the most common cause of SH
- Patients with GD rather than a TMNG as the cause of SH may be more likely to spontaneously revert
- It is important to diagnose SH; because there is 2.8 folds risk of atrial fibrillation in individuals over the age of 60 yrs, likewise post-menopausal females with SH have increased risk of fractures
Who to Treat according to the age and TSH levels:
- Individuals who are >65 years with TSH <0.1 mU/L should be treated; while 0.1-0.5 mU/L should be considered for therapy
- Individuals aged <65 years with TSH < 0.1 mU/L with heart disease, osteoporosis and hyperthyroid symptoms should be treated while with TSH 0.1-0.5 mU/L with heart disease, menopausal females and hyperthyroid symptoms should consider treatment
- Individuals with aged <65 years and asymptomatic may consider treatment with TSH <0.1 mU/L
Treatment of subclinical hyperthyroidism will depend on its cause. In the case of clinical evidence for underlying Graves’ disease (goiter, ophthalmopathy, positive TSH (receptor antibodies), low-dose thiourea therapy with methimazole could be considered as well as radioiodine.
Management of hyperthyroidism in pregnancy:
- If GD is diagnosed during 1st trimester of pregnancy, PTU is recommended treatment. Measure TRAb at diagnosis and if elevated repeat at 22-26 weeks of gestation; if thyroidectomy indicated then perform in 2nd trimester
- If it is diagnosed after 1st trimester methimazole is recommended; if surgery indicated it should be done in 2nd trimester
- Diagnosed and treated females prior pregnancy; should switch to PTU from methimazole if pregnancy is confirmed
- Once in remission after stopping ATDs, TRAb monitoring is not required
- If previous treatment with radioiodine or surgery, TRAb are measured initially and if high then repeat 22-26 weeks of gestation
Management of Graves’ ophthalmopathy (GO):
Graves’ ophthalmopathy is defined as an inflammation of the orbital and periorbital soft tissues effecting on the eye; such as pain, itching, chemosis, retraction of the eyelids, and proptosis associated with hyperactivity of thyroid gland.
Approximately 50% of Graves’ hyperthyroidism presents with Graves’ ophthalmopathy, and 5% tend to suffer from severe disease.
Assessment of the disease activity is calculated by a clinical activity score (CAS)**. The score ranges from 0-10 and also predicts response to anti-inflammatory therapy. GO is considered active if the CAS ≥3.
The severity of disease is graded as follows:
- Mild GO: Features of GO, having very mild effects on daily life, insufficient to justify treatment
- Moderate to severe GO: Features having significant effects on daily life but without sight threatening GO; sufficient to justify treatment with immunosuppressive (inactive disease) or surgical intervention (if inactive disease)
- Sight threatening GO: patients with dysthyroid optic neuropathy and/or corneal breakdown, requiring immediate intervention
** Ref: Mourits MP, Koornneef L, Wiersinga WM, et al. Clinical criteria for the assessment of disease activity in Graves’ ophthalmopathy: a novel approach. Br J Ophthalmol. 1989;73:639-644.
Therapeutic approaches to GO include:
- Local measures
- Corticosteroids
- Orbital radiation
- Surgery
Since quality of life may not be significantly improved by these measures, it is important to prevent development and/or progression of GO by identifying and treating of risk factors as follows:
- High pretreatment T3 and TRAb levels: Achieve euthyroid state as soon as possible
- Smokers: Advised to stop
- Radioiodine- induced hypothyroidism should be corrected
In patients receiving RAI for hyperthyroidism, use of glucocorticosteroids may help prevent the development of GO.
Management of drug induced thyrotoxicosis:
More than one mechanism can be induced by a single drug. Medications which may interfere and affect thyroid function to cause hyperthyroidism or thyrotoxicosis involve following mechanisms:
- Iodine induced
- Destructive thyroiditis
- Thyroid autoimmunity

Management of destructive thyroiditis:
Destructive thyroiditis results from the subacute release of preformed thyroid hormone, and is self-limited.
Common causes include:
- Subacute thyroiditis
- Painless thyroiditis (including postpartum thyroiditis, amiodarone-associated destructive thyroiditis, and lithium-associated thyroiditis)
- Acute thyroiditis- referred to suppurative thyroiditis or thyroid abscess, is usually euthyroid, occasionally may present as destructive thyrotoxicosis
a) Patients with mild symptomatic subacute thyroiditis are treated with:
- Beta-blockers
- Non-steroidal anti-inflammatory drugs (NSAIDs)
Note: Non-responders may require steroids for moderate to severe symptoms.
b) Patients with painless thyroiditis treated with:
- Beta-blockers for symptoms of thyrotoxicosis
- ATD has no role because new thyroid hormone synthesis is already low in these patients
- In severe cases, corticosteroids may be used
c) Patients with acute thyroiditis:
- Cause is usually bacterial infection requiring systemic antibiotics
- In the presence of abscess drainage is required
- Beta blocker is given for supportive therapy in case of thyrotoxicosis
- No role of ATD
Management of unusual causes of thyrotoxicosis:
As discussed earlier there are other uncommon causes that may cause hyperthyroidism or thyrotoxicosis.
 [/cq_vc_tab_item][cq_vc_tab_item tabtitle=”Medication Dose”]MEDICATIONS:
[/cq_vc_tab_item][cq_vc_tab_item tabtitle=”Medication Dose”]MEDICATIONS:
Radiopharmaceutical, Antithyroid agent
- ➣ Sodium iodide I131
Mechanism:
- Destroyed the overactive thyroid gland; brings back to normal function
- Small amount is used for diagnostic purpose by measuring the uptake by the thyroid gland
- Larger doses of sodium iodide I131 are used in certain thyroid cancer to destroy the remaining thyroid tissues after surgery
Doses:
Sodium Iodide I131
Graves’ disease
- Typically (10-15 mCi) is a sufficient single dose to achieve hypothyroid state
Toxic multinodular goiter (TMNG)
- Activity of radioiodine should be calculated according to the size of the goiter to deliver 150-200 µCi per gram of tissue corrected for 24 hrs RAIU
Toxic thyroid adenoma (TA)
- Approximately 10-20 mCi, or calculated on the bases of nodule size using 150-200 µCi per gram corrected for 24-hour RAIU
Thionamides, Antithyroid agent
- ➣ Propylthiouracil (PTU)
- ➣ Methimazole (MMI)
Mechanism
- Inhibits the conversion of iodide to iodine by binding to thyroid peroxidase
- Thus blocks synthesis of thyroxine and triiodothyronine
- Does not inactivate already formed, circulating and exogenous thyroid hormones
Doses:
Propylthiouracil (PTU)
- 50-150 mg PO TID; once the thyroid functions return to normal; it is maintained 50 mg PO TID
Thyroid storm
- Initial 500-1000 mg, then 250 mg every 4 hourly
Methimazole (MMI)
- Start 10-20 mg/day PO daily; maintenance dose: 5-10 mg once daily
Thyroid storm
- 60-80 mg/day
Drug induced thyrotoxicosis
- 20-40 mg daily
Beta-adrenergic blocking agents
β1 selectivity
- ➣ Atenolol
- ➣ Metoprolol
Nonselective
- ➣ Nadolol
- ➣ Propranolol
Mechanism:
- Blocks response to beta-adrenergic stimulation
- β1-selective beta-blocker inhibits β-adrenergic receptors within the myocardium at normal doses
- But can inhibit β-adrenergic receptors within bronchial and vascular smooth muscle at higher doses
- Nonselective beta-blocker inhibits β-adrenergic receptors within the myocardium and within bronchial and vascular smooth muscle
- Decrease heart rate and cardiac output
- Decrease renin release
Doses:
β1-selective:
Atenolol
- 25-100 mg/day PO once daily or BID; Max 200 mg/day
Esmolol
Thyrotoxicosis/thyroid storm
- IV pump 50-100 mcg/kg/min
Metoprolol
- 25-50 mg PO QID
Nonselective:
Nadolol
- 40-160 mg/day once daily
Propranolol
- Oral: 10-40 mg TID-QID
- Intravenous: 1-3 mg single dose
Thyroid storm
- Oral: 60-80 mg every 4 hrs
- Intravenous: 0.5-1 mg over 10 minutes every 3 hrs
Calcium channel blocking agents (CCBs)
Non-dihydropyridine
- ➣ Diltiazem
- ➣ Verapamil
Mechanisms:
- Blocks the inward movement of calcium by binding to the L-type calcium channels in the heart and in smooth muscle of the peripheral vasculature
- This decreases intracellular calcium leading to a reduction in muscle contraction
- Significant reduction in afterload but not preload
Doses:
Diltiazem
- 120 mg PO every 8 hrs
Verapamil
- 40-80 mg/day PO every 8 hrs
Non-steroidal anti-inflammatory drugs (NSAIDs)
- ➣ Ibuprofen
- ➣ Aspirin
Mechanism:
- Prostaglandins are believed to be a common factor in the production of pain, fever, and inflammation
- These drugs inhibit cyclooxygenase-1 and 2 (COX-1 and 2) enzymes
- This results in decreased formation of prostaglandin precursors
Doses:
Ibuprofen
- 1200 to 3200 mg/day in divided doses
Aspirin
- 2600 mg/day in divided dose
- ➣ Methylprednisolone
- ➣ Prednisone
Mechanism:
- Decreases inflammation and the normal immune response through multiple mechanisms, such as
- Inhibition of macrophage accumulation in inflamed areas
- Decreases permeability of capillary wall and edema formation
- Stabilizes leukocyte lysosomal membranes
- Also suppresses adrenal function in higher doses
Doses:
Hydrocortisone
Thyroid storm
- 300 mg IV loading dose followed by 100 mg every 8 hrs
Prednisone
Subacute thyroiditis
- 40 mg/day PO for 1-2 weeks; followed by gradual taper over 2-4 weeks or longer, depending upon clinical response
Antithyroid agent; Expectorant
- ➣ Saturated solution of potassium iodide (SSKI)
Mechanism:
- Decreases viscosity of mucus by increasing respiratory tract secretions
- Inhibits thyroid hormone synthesis and release
- Also blocks or reduces accumulation of radioactive iodine in the thyroid gland
- Antifungal activity is not known exactly
Doses
Saturated solution of potassium iodide (SSKI)
Thyroidectomy Graves’ disease
- Potassium iodide 5-7 drops (0.25-0.35 mL), or saturated solution of potassium iodide (SSKI); 1-2 drops (0.05-0.1 mL) PO TID mixed in water for 10 days prior to surgery should be given
Thyroid storm:
- 5 drops (0.25 mL or 250 mg) PO every 6 hrs
Note: Do not start until 1 hour after antithyroid drugs[/cq_vc_tab_item][/cq_vc_tabs]
Pipeline Agents
Pending new data.
Physician Resources
1. Tips for Patient Care
- Educate about importance of drug therapy compliance and surveillance for hyperthyroidism
- Avoid circumstances leading to stress
- Educate patient about thyrotoxic crisis
- Include family or social support in lifestyle modification
- Appreciate lower starting doses of drugs in elderly, debilitated and pregnant patients
- Obtain CBC/LFTs before the initiation of medication and during the first 3 months of therapy with thioamides
- Repeat thyroid test at least once a year after patient become euthyroid
- Consider concurrent risk factors and disease states with the prescribed therapy
- Amiodarone and lithium may induce hyperthyroidism
- Use beta-blockers with caution in patients with asthma and COPD
- Radioactive iodine (131I) therapy is contraindicated during pregnancy
- Propylthiouracil remains preferable to methimazole during pregnancy
- Women of childbearing age with hyperthyroidism should achieve good control prior to conception
- Cigarette smoking is a risk factor for the development and progression of Graves’ ophthalmopathy
- Radioactive iodine therapy and thyroidectomy may induce hypothyroidism
- Usually, it takes 3-4 weeks for patient to be euthyroid after the initiation of therapy
- Prognosis is good with appropriate treatment
2. Scales and Table
References
Core Resources:
- Bahn RS, Burch HB, Cooper DS, et al. Hyperthyroidism and other causes of thyrotoxicosis: Management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Endocr Pract. 2011;17:456-520
- Compendium of Pharmaceuticals and Specialties (CPS). Canadian Pharmacist Association. Toronto: Webcom Inc. 2012
- Day RA, Paul P, Williams B, et al (eds). Brunner & Suddarth’s Textbook of Canadian Medical-Surgical Nursing. 2nd ed. Philadelphia: Lippincott Williams and Wilkins; 2010
- Foster C, Mistry NF, Peddi PF, Sharma S, eds. The Washington Manual of Medical Therapeutics. 33rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010
- Gray J, ed. Therapeutic Choices. Canadian Pharmacists Association. 6th ed. Toronto: Webcom Inc. 2011
- Katzung BG, Masters SB, Trevor AJ, eds. Basic and Clinical Pharmacology. 1st ed. New York: McGraw-Hill; 2009
- Longo D, Fauci A, Kasper D, et al (eds). Harrison’s Principles of Internal Medicine. 18thed. New York: McGraw-Hill; 2011
- Management of thyroid dysfunction during pregnancy and postpartum. An endocrine society clinical practice guidelines. 2007 J Clin Endocrinol Metab:92; S1-S47
- Mechanick JI, Pessah-Pollack R, Camacho P, et al. American Association Of Clinical Endocrinologists And American College Of Endocrinology Protocol For Standardized Production Of Clinical Practice Guidelines, Algorithms, And Checklists – 2017 Update. Endocrine Practice:2017, Vol. 23, No. 8, pp. 1006-1021. doi: 10.4158/EP171866.GL
- McPhee SJ, Papadakis MA, eds. Current Medical Diagnosis & Treatment. 49th ed. New York: McGraw-Hill; 2010
- Pagana KD, Pagana TJ eds. Mosby’s Diagnostic and Laboratory Test Reference. 9th ed. St. Louis: Elsevier-Mosby; 2009
- Ross DS, Burch HB, Cooper DS, et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid. 2016;26:1343-1421. DOI: 10.1089/thy.2016.0229
- Skidmore-Roth L. ed. Mosby’s drug guide for nurses. 9th ed. St. Louis: Elsevier-Mosby; 2011
- Skidmore-Roth L, ed. Mosby’s nursing drug reference. 24th ed. St. Louis: Elsevier-Mosby; 2011
- Suwansaksri N, Preechasuk L, Kunavisarut T. Nonthionamide Drugs for the Treatment of Hyperthyroidism: From Present to Future. International Journal of Endocrinology. 2018. 1-10. 10.1155/2018/5794054. https://doi.org/10.1155/2018/5794054
Online Pharmacological Resources:
- e-Therapeutics
- Lexicomp
- RxList
- Epocrates
Journals/Clinical Trials:
- Assessment of iodine deficiency disorders and monitoring their elimination. A guide for programme managers, (3rd edition). World Health Organization 2007
- Aoki Y, Belin RM, Clickner R, Jeffries R, Phillips L, Mahaffey KR. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999-2002). Thyroid. Dec 2007;17(12):1211-23
- Abraham P, Avenell A, Park CM, et al. A systematic review of drug therapy for Graves’ hyperthyroidism. Eur J Endorinol. 2005;153:489-498
- Boelaert K, Torlinska B, Holder RL, Franklyn JA. Older subjects with hyperthyroidism present with a paucity of symptoms and signs: a large cross-sectional study J Clin Endocrinol Metab. 2010; 95:2715-26
- Bogazzi F, Giovannetti C, Fessehatsion R, et al. Impact of lithium on efficacy of radioactive iodine therapy for Graves’ disease: a cohort study on cure rate, time to cure, and frequency of increased serum thyroxine after antithyroid drug withdrawal. J Clin Endocrinol Metab. 2010;95: 201-208
- Collet T-H, Gussekloo J, Bauer DC, et al. Subclinical Hyperthyroidism and the Risk of Coronary Heart Disease and Mortality Archives of Internal Medicine 2012; 172:799-809. doi:10.1001/archinternmed.2012.402
- Jumaily JS, Noordzij JP, Dukas AG, et al. Prediction of hypocalcemia after using 1- to 6-hour postoperative parathyroid hormone and calcium levels: an analysis of pooled individual patient data from 3 observational studies. Head Neck. 2010;32:427-434
- Kikuchi S, Noguchi S, Yamashita H, et al. Prognosis of small thyroid cancer in patients with Graves’ disease. Br J Surg. 2006; 93:434-439
- Mazza E, Carlini M, Flecchia D, et al. Long-term follow-up of patients with hyperthyroidism due to Graves’ disease treated with methimazole. Comparison of usual treatment schedule with drug discontinuation vs continuous treatment with low methimazole doses: a retrospective study. J Endocrinol Invest. 2008;31:866-872
- Metso S, Auvinen A, Huhtala H, et al. Increased cancer incidence after radioiodine treatment for hyperthyroidism. Cancer 2007; 109:1972-9
- Nakamura H, Noh JY, Itoh K, et al. Comparison of methimazole and propylthiouracil in patients with hyperthyroidism caused by Graves’ disease. J Clin Endocrinol Metab. 2007;92:2157-2162
- Peters H, Fischer C, Bogner U, et al. Treatment of Graves’ hyperthyroidism with radioiodine: results of a prospective randomized study. Thyroid. 1997;7:247-251
- Roh JL, Park CI. Routine oral calcium and vitamin D supplements for prevention of hypocalcemia after total thyroidectomy. Am J Surg. 2006;192:675-678
- Ruiz JK, Rossi GV, Vallejos HA, et al. Fulminant hepatic failure associated with propylthiouracil. Ann Pharmacother. 2003;37:224-228
- Tajiri J, Noguchi S, Murakami T, et al. Antithyroid drug-induced agranulocytosis. The usefulness of routine white blood cell count monitoring. Arch Intern Med. 1990;150:621-624
