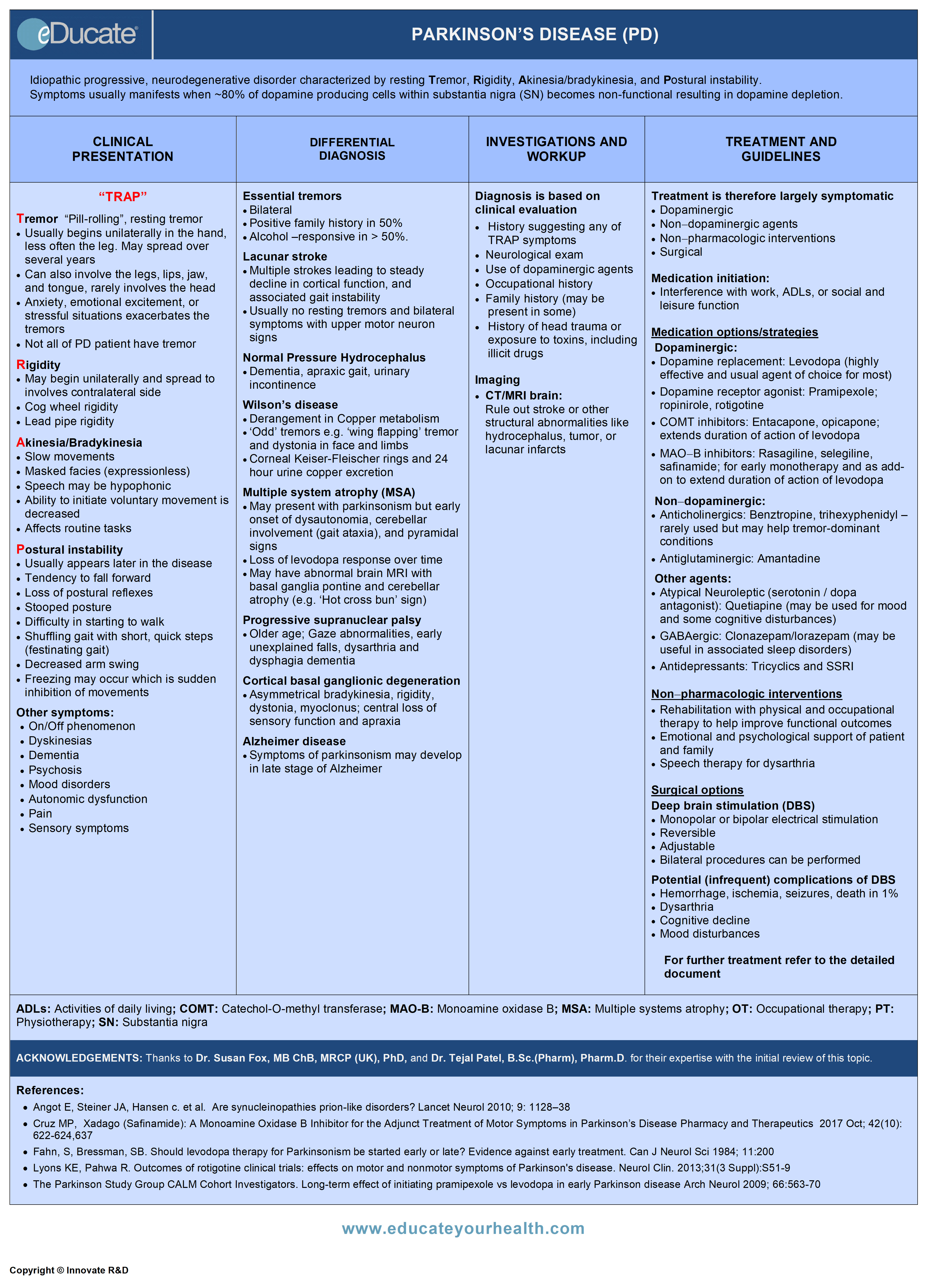
Parkinson’s Disease (PD)
ACKNOWLEDGEMENTS:
Thanks to Dr. Susan Fox, MB ChB, MRCP (UK), PhD, Associate Professor of Neurology, University Health Network, Toronto Western Hospital, University of Toronto, and Dr. Tejal Patel, B.Sc.(Pharm), Pharm.D., Clinical Assistant Professor, School of Pharmacy, University of Waterloo, ON Canada for their expertise with the initial review of this topic.
[pdf-embedder url=”https://www.educateyourhealth.com/wp-content/uploads/2020/06/PD-Brochure.pdf” width=”750″]
Definition
Idiopathic progressive, neurodegenerative disorder characterized by resting Tremor, Rigidity, Akinesia/bradykinesia, and Postural instability (‘TRAP’). Symptoms usually manifest when ~80% of dopamine-producing cells within substantia nigra (SN) becomes non-functional resulting in dopamine depletion.
Etiology
Proposed Classification Scheme for Parkinsonism. (Ref: Galpern WR, Lang, AE Ann Neurol 2006; 59:449-458)
Classical Parkinsonism
- Sporadic
- Idiopathic Parkinson’s disease
- Genetic
- LRRK2 mutations
- SNCA mutations, duplications
- Parkin mutations
- PINK1 mutations
- DJ-1 mutations
- GBA mutations
- Others (e.g. SCA2)
Atypical Parkinsonism
- Sporadic
- Dementia with Lewy bodies
- Multiple system atrophy
- Progressive supranuclear palsy
- Corticobasal degeneration
- Others
- Genetic
- Frontotemporal dementia and Parkinsonism linked to chromosome 17 (FTDP-17)
- Others
- Wilsons disease
- Huntington’s disease
Secondary Parkinsonism
- Drug-induced
- Toxin-induced
- Vascular/infection
Environmental risk factors for idiopathic PD
- Unknown, but the following have been suggested as associated with increased risk:
- Environmental toxins
- Pesticides/herbicides
- Head trauma
- Environmental factors that have been suggested to have a protective effect
- Smoking
- Caffeine
- Elevated urates
- Use of non-steroidal anti-inflammatory drugs
Epidemiology
Canada:
- Prevalence: Approx. 160 per 100,000
- Incidence: 10 to 20/100,000/year
Both prevalence and incidence increase with age.
- 85% diagnosed are above the age 65 years
- Approx. 100,000 Canadians affected
- Approx. 6.3 million worldwide
Pathophysiology
- Disease probably begins in the olfactory tract and brain stem (early loss of sense of smell) and spreads rostrally and caudally to substantia nigra and eventually to cortical regions
- Loss of dopaminergic neurons in the substantia nigra leads to dopamine depletion within the basal ganglia. Multiple neurotransmitter abnormalities occur in the basal ganglia circuitry resulting in motor symptoms of PD
- Non-motor symptoms of PD, such as neuropsychiatric, autonomic, sleep and pain likely result from degeneration of cortical and other brainstem regions
- Observed pathological abnormalities in patients with PD include:
- Degeneration of neurons in (SN)
- Presence of Lewy bodies correlates with symptoms
- Exact mechanisms of neurodegeneration are unknown, likely involves genetic and environmental factors
- Mitochondrial dysfunction and oxidative stress as well as misfolding and aggregation of proteins (alpha synuclein) appears to underlie the neurodegenerative process
- There is growing evidence to suggest a potential link along the gut-brain axis in the pathogenesis of Parkinson’s Disease, whereby disturbances in the gut biome facilitates neuronal toxicity that contribute to increased aberrant alpha synuclein production and subsequent injury to central neurons and co-ordinated cell death involving the brain stem, nigrostriatal and other pathways that manifest the clinical symptoms of Parkinson’s Disease

Clinical Presentation
1. Motor Symptoms
Tremor
“Pill-rolling”, resting tremor
- Usually begins unilaterally in the hand, less often the leg. May spread over several years
- Tremor can also involve the legs, lips, jaw, and tongue, Rarely involves the head
- Anxiety, emotional excitement, or stressful situations exacerbate the tremors
- Not all patients with PD develop tremor

Rigidity
- May begin unilaterally and spread to involve contralateral side
- Cogwheel rigidity due to overlay of tremor
- Lead pipe rigidity is the increased tone that is present throughout the range of movement
- Will increase with activation (ask subject to open and close opposite hand)
Akinesia / Bradykinesia
- Slow movements
- Masked facies (expressionless), decreased blink
- Speech may be hypophonic, stuttering or dysarthria
- Ability to initiate voluntary movement is decreased
- Affects routine tasks (buttoning, writing (micrographia), typing, tying knots, difficulty rising from seated position)
Postural instability and gait dysfunction
- Usually appears later in the disease course, but may occur early in some
- Tendency to fall forward (propulsion) or backward (retropulsion)
- Loss of postural reflexes
- Stooped posture
- Difficulty in starting to walk, turning, and stopping
- Shuffling gait with short, quick steps (festinating gait)
- Decreased arm swing
- Freezing may occur which is sudden inhibition of movements
OTHER SYMPTOMS
Motor fluctuations
- On/Off phenomenon:
- May occur with advancing disease due to disease progression and chronic levodopa treatment
- Periods of satisfactory or good control (on effect) is followed by periods of near immobility (off effect)
- Symptoms of wearing off are apparent when the duration of levodopa effect subsides
- Dyskinesia:
- Over time many patients with PD may develop medication-induced dyskinesia some of which can be disabling
- Many PD patients may be either unaware of dyskinesia or not have any functional disability with the involuntary movements
Musculoskeletal problems
- Camptocormia (bent-spine)
- Head drop (less common)
- Kyphosis
2. Non-Motor Symptoms
Dementia
- Dementia can occur later in the course of disease in 10-15% patients
- Short-term memory and visuoperceptual functions may be affected
Psychosis
- Sense of presence: Well formed visual and less commonly auditory and tactile hallucinations
- Paranoid delusions: May be associated with cognitive impairment; Can be triggered by dopaminergic drugs; always rule out other causes of delirium e.g. infection
Mood disorders
- Depression
- Anxiety
- Apathy
- All very common in PD. Maybe ‘premotor’ features i.e. occur years before the onset of PD
Sleep disorders
- Excessive daytime sleepiness
- Insomnia
- REM sleep behaviour disorder (maybe a ‘premotor’ feature)
- Sleep disorders are very common and are often drug-induced
Autonomic dysfunction
- Urinary frequency and nocturia
- Constipation
- Postural hypotension
Pain
- Can be multifactorial due to rigidity or dystonia or ‘central’
- Sensory symptoms-Numbness/tingling
Differential Diagnosis
Essential tremors
- Bilateral, no rest component in tremors in the early stage of disease
- Positive family history in 50%
- Alcohol-responsive in >50%
Lacunar stroke
- Multiple strokes leading to steady decline in cortical function, and associated gait instability (apraxic gait), rigidity, dysarthria
- Usually no resting tremors and bilateral symptoms with upper motor neuron signs (sometimes called ‘Lower body Parkinson’s disease)
Normal pressure hydrocephalus
- Dementia, apraxic gait (wide-based small steps, normal arm swing), urinary incontinence (needs CT/MRI brain scan)
Wilson’s disease
- Derangement in Copper metabolism
- ‘Odd’ tremors e.g. ‘wing flapping’ tremor and dystonia in face and limbs
- Check for corneal Keiser-Fleischer rings and 24h urinary copper excretion
Multiple system atrophy (MSA)
- May present with parkinsonism but early onset of dysautonomia, cerebellar involvement (gait ataxia), and pyramidal signs
- Loss of levodopa response over time
- May have abnormal brain MRI with basal ganglia and cerebellar atrophy (e.g. ‘Hot cross bun’ sign)
Progressive supranuclear palsy
- Older age, gaze abnormalities, early unexplained falls, dysarthria, dysphagia, and dementia
Cortical-basal ganglionic degeneration
- Asymmetrical bradykinesia, rigidity, dystonia, myoclonus, central loss of sensory function and apraxia
Alzheimer disease
- Symptoms of parkinsonism may develop in the late stage of Alzheimer
Investigation and Workup
Diagnosis of PD is based upon clinical evaluation
- Thorough history of presenting complaints
- Family and occupational history
- Neurological exam
- Response to dopaminergic therapy
- Neuroimaging-CT/ MRI brain: Rule out structural abnormalities such as hydrocephalus, tumour, or lacunar infarcts
Treatment
- Dopaminergic
- Non-dopaminergic agents
- Non-pharmacologic interventions
- Surgical
1. MEDICATION INITIATION:
Indications
- Interference with work, activities of daily living (ADL), or social and leisure function
- Gait disturbance, falls
- Patient’s preferences-control of non-disabling but socially embarrassing condition such as tremor
Medication options/strategies:
There are a few options available for the treatment of Parkinson’s. Of these, levodopa may be the most efficacious, but the potential for developing disabling dyskinesias, often leads to trial of other agents, particularly in younger individuals. Consequently, there is no standardized approach with a “first line” treatment as such, and treatment is often individualized.
Dopaminergic:
- Dopamine replacement: Levodopa (highly effective and usual agent of choice for most)
- Dopamine receptor agonist: Pramipexole, ropinirole, rotigotine patch
- COMT inhibitors:
- Entacapone: extends the duration of action of levodopa
- Opicapone: high binding affinity with the COMT enzyme and strong inhibition over 24 hours – promising data
- MAO-B inhibitors: Rasagiline, selegiline, safinamide; for early monotherapy and as an add-on to extend the duration of action of levodopa
Non-dopaminergic:
- Anticholinergics: Benztropine; trihexyphenidyl-rarely used but may help tremor-dominant conditions
- Antiglutaminergic: Amantadine (most helpful as a treatment for dyskinesia but can have mild antiparkinsonian effects)
Other agents for non-motor symptoms:
- Low dose atypical neuroleptic: (serotonin/dopamine antagonist): Quetiapine and clozapine (with CBC monitoring) for visual hallucinations and psychosis
Note: Typical neuroleptics e.g. haloperidol should not be used in PD due to the risk of worsening motor function, atypical neuroleptics e.g. olanzapine and risperidone should be used cautiously.
- Clonazepam/lorazepam-may be useful in associated sleep disorders e.g. REM sleep behaviour disorder (RBD), and anxiety
- Antidepressants: Tricyclics and SSRI, for anxiety and depression
- Melatonin-for insomnia, and RBD
- Anticholinergics e.g. oxybutynin-for overactive bladder syndrome
- Stool softeners and laxatives-for constipation
- Analgesic use as required for musculoskeletal discomfort
- Ipratropium spray, sublingual atropine drops-administered topically for drooling saliva
2. NON-PHARMACOLOGIC INTERVENTIONS
- Rehabilitation with physical and occupational therapy to help improve functional outcomes in subjects with specific gait and balance problems
- Emotional and psychological support of patient and family
- Speech therapy for dysarthria
- Adaptive strategies → adjustments in their homes to reduce the risk of falls and assist mobility; use of cane or walker when required
- Providing resources about support groups, counsellors, societies
3. SURGICAL OPTIONS
Deep brain stimulation (DBS)
- Selection criteria for suitable patients are essential. Usually <70 years old; must be levodopa responsive and have no cognitive problems
- Procedure is reversible
- Adjustable for optimal benefit vs side-effects
- Bilateral procedures are usually performed if STN is the target
- Following types of stimulation can be done
- Thalamic stimulation: Relieves tremors only (may be a unilateral procedure)
- Subthalamic nucleus (STN) stimulation: Relieves tremors, rigidity, dyskinesias, and motor symptoms (commonest target)
- Unilateral pallidal stimulation: Relieves dyskinesia (rarely used)
- Potential (infrequent) complications of DBS
- Hemorrhage, ischemia, seizures, death in 1%
- Dysarthria
- Cognitive decline
- Mood disturbances
[/cq_vc_tab_item][cq_vc_tab_item tabtitle=”Medication Dose”]MEDICATIONS:
Dopamine precursor/Dopamine decarboxylase inhibitor
- Levodopa/Carbidopa
- Levodopa/Benserazide
- Levodopa/Carbidopa/Entacapone
Mechanism
- Dopamine replacement therapy
- Carbidopa and Benserazide inhibit peripheral breakdown of Levodopa (L-Dopa) by inhibiting decarboxylation, thereby increasing the availability of L-Dopa to cross the blood-brain barrier (BBB)
- Within the basal ganglia, L-Dopa is then converted to dopamine
Dose:
Levodopa/Carbidopa:
Immediate Release (100/10; 100/25; 250/25)
- Start 100/10 mg or 100/ 25 mg PO ½-1 tab TID; Adjust dose according to response, increase 100/25 mg (1 tablet) PO every 7 days or 250/25 mg (1/2-1 tablet) PO every 7 days
- Most will get benefit with 400-600 mg levodopa. No maximum dose but unusual to require >2 g/d
Extended-release (100/25; 200/50)
- Can use instead of IR however no real advantage as slower, less predictable time of onset. Maybe useful at bedtime for nocturnal akinesia
- Start 200/50 mg PO BID, then adjust dose according to response, increase dose by 0.5-1 tab/day every 3 days giving dose interval of at least 4-8 hours during the waking day; Max. 600 mg/2400 mg/day
Levodopa/Benserazide (50-12.5; 100-25; 200-50)
- 100/25 mg PO once daily or BID; May increase every 3-4 days until optimal effect is achieved; optimal dosage 400/100 mg to 800/200 mg/day divided into 4-6 doses
- Note:
- May use 200/50 mg form for maintenance therapy once the optimal dosage has been determined
- May use 50-12.5 mg form when frequent dosing is required to minimize adverse effects
Caveat: Simultaneous ingestion of L-dopa with high protein meals could decrease absorption and may impact on efficacy
Anti-Parkinson’s agent/COMT inhibitor
- Entacapone
- Opicapone
Mechanism
- Entacapone: Reversible and selective inhibitor of peripheral catechol-O-methyltransferase (COMT); multiple doses per day
- Opicapone: high binding affinity with the COMT enzyme and strong inhibition; once per day dosing
- When administered with Levodopa, they decrease the peripheral degradation of Levodopa via the COMT mediated pathways, leading to more sustained Levodopa serum levels
- Enhanced serum concentrations of L-Dopa increases availability for traversing the blood-brain barrier
Dose:
Entacapone:
- 200 mg PO with each dose of Levodopa/Carbidopa or Levodopa/Benserazide, Maximum up to 8 times/day (1600 mg/day)
- Note: May require an average decrease of 25% in the daily Levodopa dose if the patient is taking Levodopa ≥800 mg/day or had moderate-to-severe dyskinesias before beginning therapy
Opicapone:
- A newer COMT inhibitor; Typical dose: 50 mg PO once daily at bedtime
Combination Therapy:
Anti-Parkinsonian dopaminergic agent
- Levodopa/Carbidopa/Entacapone
Mechanism
- Dopamine replacement therapy/Catechol-O-methyltransferase (COMT)
Dose:
Carbidopa/Levodopa/Entacapone (Stalevo)
- Therapy should be individualized and adjusted according to the desired therapeutic response
- The combination is supplied as tablets in 6 different strengths

Anti-Parkinson’s agent/Monoamine oxidase (MAO) B inhibitor
- Rasagiline
- Safinamide
- Selegiline
Mechanism
- Selective irreversible Monoamine Oxidase B (MAO-B) Inhibitors
- Inhibition of MAO-B prevent breakdown of dopamine
- May also increase dopaminergic activity by interfering with dopamine reuptake at the synapse
Dose:
Rasagiline:
- Oral 1 mg once daily; may have to reduce levodopa dose if taken concurrently
Safinamide:
- 50 mg orally once a day initially. May increase to 100 mg orally once a day after two weeks as required, and as tolerated. based on individual need and tolerability; Maximum dose: 100 mg per day
Selegiline:
- Starting dose 2.5 mg PO BID, then 5 mg PO BID daily with meals. Maintenance dose 5 mg PO BID
- Renal impairment
- no change
- Liver disease
- Moderate: 50 mg daily
- Severe: contraindicated
Anti-Parkinson’s agent, Dopamine agonist
- Pramipexole
- Ropinirole
Mechanism
- Activates postsynaptic dopamine receptors in the striatum and substantia nigra
- Longer duration of action compared to levodopa
- No effect of food on absorption via GI tract
Dose:
Pramipexole:
Immediate-release form
- Initial 0.125 PO TID; may increase dose gradually every 5-7 days; usual range 1.5-4.5 mg/day divided TID; Max. 4.5 mg/day
- Renal impairment
- Clcr 35-59 mL/minute: Initial: 0.125 mg BID; Max 1.5 mg BID
- Clcr 15-34 mL/minute: Initial: 0.125 mg once daily; Max.1.5 mg once daily
Extended-release form-(USA)
- Initial: 0.375 mg PO once daily; increase gradually to 0.75 mg once daily; Max. 4.5 mg once daily
- Renal impairment:
- Clcr >50 mL/min no dose adjustment required
- Clcr 30-50 mL/min: Initial: 0.375 mg every other day; may increase to 0.375 mg/dose after 1 week and every week as required; Max: 2.25 mg once daily
Ropinirole:
Immediate-release form
- 0.25 mg PO (TID); increase by 0.25 mg/dose every 7 days; daily dose may be increased by 0.5 to 1.0 mg/dose after a month until the desired response is achieved; Max. 24 mg/day
Rotigotine:
- Initially 2 mg/24 hours and increase by 2 mg/24 hours every 1 -2 weeks if required and as tolerated to a maximum 8 mg/24 hours
Anti-Parkinson’s agent/NMDA receptor inhibitor
- Amantadine
Mechanism
Possibly enhances dopamine action by:
- Blocking the reuptake of dopamine into presynaptic neurons or by increasing dopamine release from presynaptic fibers
- Reduces levodopa-induced dyskinesia by antagonism of NMDA glutamate receptors
Dose:
Amantadine:
- Usual dose 100 mg PO BID; Max. 400 mg/day in divided dose
- Note: Start 100 mg/day once daily in Patients taking another anti-Parkinson drug or debilitated; may increase dose to 100 mg twice daily after 1-2 weeks, if needed
- Renal impairment:
- Clcr 30-50 mL/min: 200 mg on day 1, then 100 mg PO once daily
- Clcr 15-29 mL/min: 200 mg on day 1, then 100 mg PO every other day
- Clcr <15 mL/min: 200 mg every week
Anti-Parkinson’s agent/Anticholinergic agent
- Benztropine
- Trihexyphenidyl
Mechanism
- Possesses both anticholinergic and antihistaminic effects
- May also inhibit reuptake and storage of dopamine, thereby prolonging the synaptic action of dopamine
Dose:
Benztropine:
- 0.5-1 mg PO/IM/IV once daily at night; increase dose every 5-6 days; usual dose 1-2 mg/day in two divided doses; Max. 6 mg/da
Trihexyphenidyl:
- 1 mg PO QID on the first day; then increase by 2 mg every 3-5 days; usual dose 6-10 mg/day in divided doses; Max. 15 mg/day
[/cq_vc_tab_item][/cq_vc_tabs]
Clinical Trials
- Levodopa and the Progression of Parkinson’s Disease
- A Double-Blind, Delayed-Start Trial of Rasagiline in Parkinson’s Disease
- A Five-Year Study of the Incidence of Dyskinesia in Patients with Early Parkinson’s Disease Who Were Treated with Ropinirole or Levodopa
- Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson’s disease (PD SURG trial)
- Rivastigmine for Dementia Associated with Parkinson’s Disease
- Long-term effect of initiating pramipexole vs levodopa in early Parkinson disease (CALM-PD)
Physician Resources
1. Tips for patient care
General management:
- Medication may not be indicated in early stages
- Encourage exercise and a healthy lifestyle
- Communication with patients and families is paramount. Both oral and written communication may be helpful throughout the course of the disease, as patient with PD may develop cognitive impairment, a communication deficit and/or depression
- Depression is very common in all stages of PD and is often missed due to the clinical features of the disease. Anxiety is also common; the patient who is frequently calling the office is likely anxious. Treatment of anxiety and depression through counselling, or appropriate medications is often beneficial
- Inform patients and families about potential sleeping problems such as insomnia or REM sleep behaviour disorder
- Patients with PD who have sudden onset of sleep (usually medication induced) should be cautioned and advised not to drive. Document sleep disturbances using the Epworth Sleepiness Scale (http://epworthsleepinessscale.com)
- As the disease progresses patients and families need to be aware of:
- Fall risk-encourage the use of canes and walkers where appropriate
- Remove rugs and avoid uneven floors
- Nearing end-stage be aware of
- – Aspiration risk and ensuing pneumonia
- – Bedsores (due to immobility)
- Suggest high fluid intake and a high-fiber diet to prevent or treat constipation
- Patients should be encouraged to keep follow-up visit with a Neurologist
Medications:
- Most drug therapy is required when symptoms interfere with everyday life
- Use lower starting doses in elderly and debilitated patients
- Treatment should be titrated slowly to avoid unnecessary side effects
- Advise patient to establish prescribed routine for pill-taking
- Evaluate cost and affordability and insurance coverage for patients
- Consider concurrent risk factors and disease states with the prescribed therapy
- Monitor patient regularly to adjust medications as required
- The management of depression in people with PD should be individualized, and needs to take into account their co-existing therapy
- Be aware of impulse control disorder (ICD) (abnormal behaviours, such as hypersexuality, pathological gambling, compulsive eating (usually sweets) that can occur in up to 15% of patients treated with a dopamine agonist. People at risk are younger, male, often with preexisting or family history of addiction. Always ask about ICD as patients and family members may not volunteer this information
- Adherence to treatment should be assessed at each visit
- Psychosis is due to the disease itself and may be exacerbated by drugs but remember to exclude reversible causes such as intercurrent infections etc.
Social and Stress factors:
- Ensure patient and family are well informed about disease and its treatment
- Include family or social support in lifestyle modification
- Patient and family should be made aware of local support groups as well as online information
Activities (Physical, Mental, others):
- Stress the importance of staying active and having a regular exercise routine
- Occupational therapy should be available for people with PD
- Speech therapy indicated and helpful in dysarthria or hypoarthria
- Advise patients to keep themselves mentally active
2. Scales and Tables:
References
Core Resources:
- Brust JCM (2007) Current Diagnosis and Treatment (Neurology) (2nd ed.) New York: McGraw Hill
- Compendium of Pharmaceuticals and Specialties (CPS). Canadian Pharmacist Association. Toronto: Webcom Inc. 2012
- Day RA, Paul P, Williams B, et al (eds). Brunner & Suddarth’s Textbook of Canadian Medical-Surgical Nursing. 2nd ed. Philadelphia: Lippincott Williams and Wilkins; 2010
- Foster C, Mistry NF, Peddi PF, Sharma S, eds. The Washington Manual of Medical Therapeutics. 33rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010
- Gray J, ed. Therapeutic Choices. Canadian Pharmacists Association. 6th ed. Toronto: Webcom Inc. 2011
- Grimes D, Gordon J, Snelgrove B. et al. Canadian Guidelines on Parkinson’s Disease. Can J Neurol Sci. 2012;39: S1-S30
- Katzung BG, Masters SB, Trevor AJ, eds. Basic and Clinical Pharmacology. 11th ed. New York: McGraw-Hill; 2009
- Longo D, Fauci A, Kasper D, et al (eds). Harrison’s Principles of Internal Medicine. 18th ed. New York: McGraw-Hill; 2011
- McPhee SJ, Papadakis MA, eds. Current Medical Diagnosis & Treatment. 49th ed. New York: McGraw-Hill; 2010
- Pagana KD, Pagana TJ eds. Mosby’s Diagnostic and Laboratory Test Reference. 9th ed. St. Louis: Elsevier-Mosby; 2009
- Rowland LP et al. (2010) Merritt’s Neurology (9th ed.) Philadelphia: Lippincott Williams and Wilkins
- Skidmore-Roth L. ed. Mosby’s drug guide for nurses. 9th ed. St. Louis: Elsevier-Mosby; 2011
- Skidmore-Roth L, ed. Mosby’s nursing drug reference. 24th ed. St. Louis: Elsevier-Mosby; 2011
- National Collaborating Centre for Chronic Conditions. Parkinson’s disease: national clinical guideline for diagnosis and management in primary and secondary care. London: Royal College of Physicians, 2006
Online Pharmacological Resources:
- e-Therapeutics
- Lexicomp
- RxList
- Epocrates
Journals/Clinical Trials:
- Angot E, Steiner JA, Hansen c. et al. Are synucleinopathies prion-like disorders? Lancet Neurol 2010; 9: 1128–38
- Block, G, Liss, C, Reines, S, et al. Comparison of immediate-release and controlled-release carbidopa/levodopa in Parkinson’s disease: A multicenter 5-year study. Eur Neurol 1997; 37:23
- Cruz MP, Xadago (Safinamide): A Monoamine Oxidase B Inhibitor for the Adjunct Treatment of Motor Symptoms in Parkinson’s Disease Pharmacy and Therapeutics 2017 Oct; 42(10): 622-624,637
- Emre M, Aarsland D, Albanese A, Rivastigmine for Dementia Associated with Parkinson’s Disease N Engl J Med 2004; 351:2509-2518
- Fahn, S, Bressman, SB. Should levodopa therapy for Parkinsonism be started early or late? Evidence against early treatment. Can J Neurol Sci 1984; 11:200
- Galpern WR, Lang, AE Interface between tauopathies and synucleinopathies: A tale of two proteins. Ann Neurol 2006; 59:449-458
- Goedert, M., Spillantini, M. G., Del Tredici, K. & Braak, H. 100 years of Lewy pathology. Nature Rev. Neurol 2012; 9, 13–24
- Hauser RA, Cantillon M, Pourcher E et al. Preladenant in patients with Parkinson’s disease and motor fluctuations The Lancet Neurology 2011; 10: 221-229
- Hauser, RA, McDermott, MP, Messing, S. Factors associated with the development of motor fluctuations and dyskinesias in Parkinson disease. Arch Neurol 2006; 63:1756
- Irwin DJ, Lee VM, Trojanowski JQ. Parkinson’s disease dementia: convergence of α-synuclein, tau and amyloid-β pathologies. Nat Rev Neurosci. 2013;14(9):626-636. doi:10.1038/nrn3549
- Kumar, N, Van Gerpen, JA, Bower, JH, Ahlskog, JE. Levodopa-dyskinesia incidence by age of Parkinson’s disease onset. Mov Disord 2005; 20:342
- Lyons KE, Pahwa R. Outcomes of rotigotine clinical trials: effects on motor and nonmotor symptoms of Parkinson’s disease. Neurol Clin. 2013;31(3 Suppl): S51-9
- Marks MJ, Bartus RT, Siffert J, et al. Gene delivery of AAV2-neurturin for Parkinson’s disease The Lancet Neurology 2010; 9: 1164-1172
- Mizuno Y, Hasegawa K, Kondo T et al. Clinical efficacy of istradefylline (KW-6002) in Parkinson’s disease Mov Disord. 2010; 25:1437-43
- Olanow C W, Rascol O, Hauser R, et al, A Double-Blind, Delayed-Start Trial of Rasagiline in Parkinson’s Disease ADAGIO Study Investigators N Engl J Med 2009; 361:1268-1278
- Postuma, RB, Lang, AE. Homocysteine and levodopa: should Parkinson disease patients receive preventative therapy? Neurology 2004; 63:886
- Rascol O, Brooks DJ, Korczyn AD, et al, A Five-Year Study of the Incidence of Dyskinesia in Patients with Early Parkinson’s Disease Who Were Treated with Ropinirole or Levodopa. 056 Study Group.N Engl J Med 2000; 342:1484-1491
- Sampson TR, Debelius JW, Thron T, et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell. 2016;167(6):1469-1480.e12. doi:10.1016/j.cell.2016.11.018
- Shrivastava A The hot cross bun sign Radiology 2007, 245: 606-607
- The Parkinson Study Group CALM Cohort Investigators. Long-term effect of initiating pramipexole vs levodopa in early Parkinson disease Arch Neurol 2009; 66:563-70
- The Parkinson Study Group A Controlled Trial of Rotigotine Monotherapy in Early Parkinson’s Disease. Arch Neurol. 2003;60 (12):1721-1728
- The Parkinson Study Group. Levodopa and the Progression of Parkinson’s Disease N Engl J Med 2004; 351:2498-2508
- The Parkinson Study Group. Impact of deprenyl and tocopherol treatment on Parkinson’s disease in DATATOP patients requiring levodopa. Ann Neurol 1996; 39:37-45
- Williams A, Gill S, Varma T, et al, for the PD SURG Collaborative Group Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson’s disease (PD SURG trial) The Lancet Neurology 2010; 9:581-591
