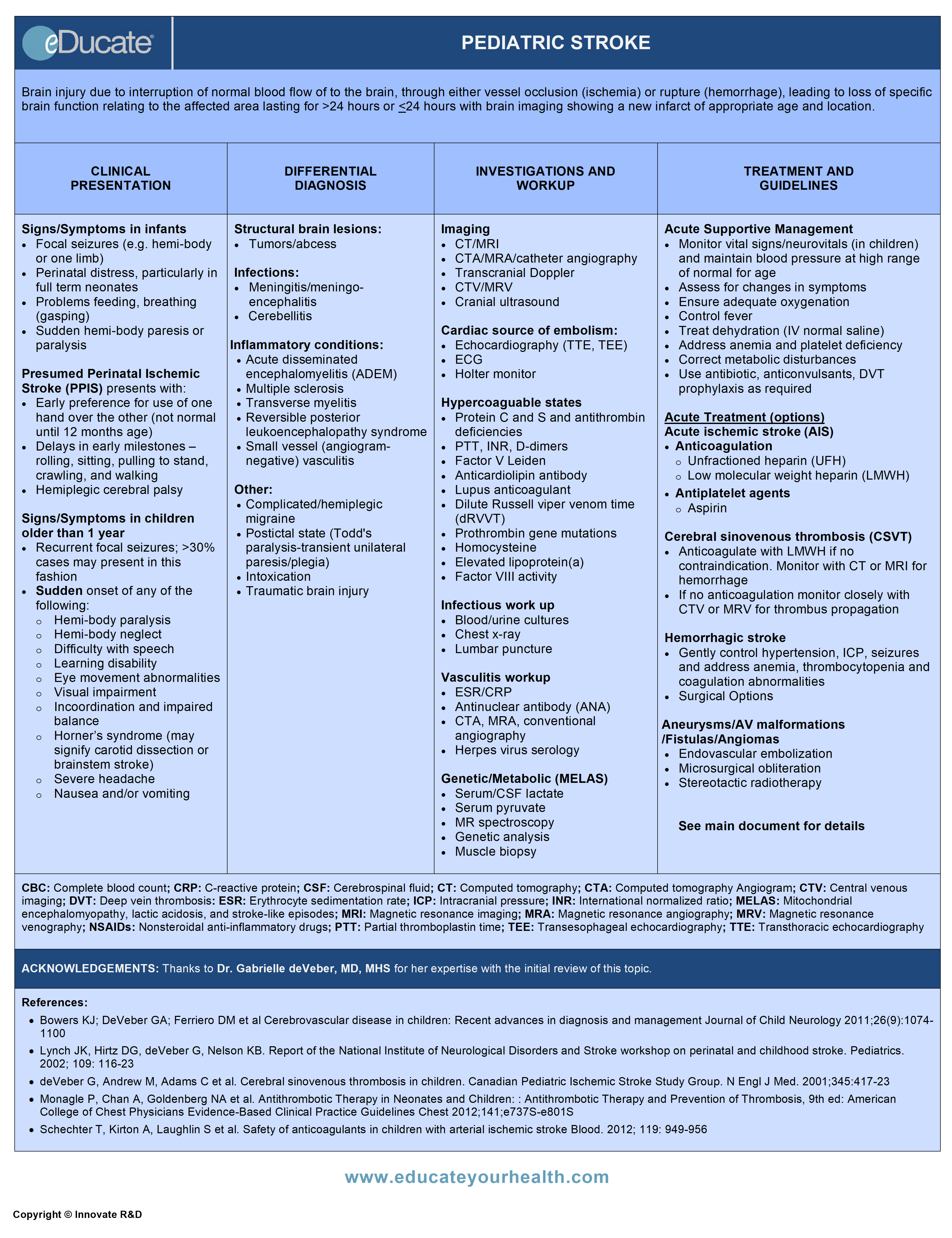
Pediatric Stroke
ACKNOWLEDGEMENTS:
Thanks to Dr. Gabrielle deVeber, MD, MHS, Associate Professor, Pediatrics, University of Toronto, Scientist, Hospital for Sick Children Research Institute, Director, Children’s Stroke Program, Division of Neurology, Hospital for Sick Children, Toronto, ON Canada for her expertise with the initial review of this topic.
Definition
Brain injury due to interruption of normal blood flow to the brain, through either vessel occlusion (ischemia) or rupture (hemorrhage), leading to loss of specific brain function relating to the affected area lasting for >24 hours (WHO) or ≤24 hours with brain imaging showing a new infarct of appropriate age and location.
Pediatric stroke can occur in two phases:
Perinatal phase: 20th week of gestation to 28th day after birth.
- Prenatal phase: 20th week to birth
- Newborn phase: Birth to 28th day
Acute Neonatal Stroke:
- Perinatal stroke can be acutely diagnosed as newborn stroke (birth to 28th day)
Presumed Perinatal Ischemic Stroke (PPIS):
- Perinatal stroke can be diagnosed later in infancy when hemiparesis emerges gradually with maturation and imaging demonstrates remote infarct presumed to have occurred in the perinatal period
Childhood phase: 29th day of birth to 18 years
Etiology
- Approximately 1/4th-1/3rd of all childhood strokes are idiopathic (40% of perinatal and 10% of childhood)
- As with adults, may be either ischemic or hemorrhagic
Hemorrhagic Stroke (~45%)
Primary mechanisms/causes:
- Cerebral sinovenous thrombosis (CSVT)
- Vascular malformation
- Brain tumour
- Periventricular hemorrhagic infarction
- Rupture of an arterial aneurysm
- Drug or alcohol abuse by the mother
- Neonatal alloimmune thrombocytopenia (NAIT)
Ischemic Stroke (~55%)
Primary mechanisms/causes:
- Cerebral Sinovenous Thrombosis (CSVT)
- Incidence of CSVT is 0.7 in 100,000 children (Canadian Registry)
- In the Canadian Pediatric Ischemic Stroke Registry, 6% of children died after ischemic stroke due to CSVT, 72% had residual neurologic deficits, and 22% fully recovered
Ref: deVeber G et al. N Engl J Med. 2001; 345:417-23
- Prothrombotic disorders (20-50%)
- Protein C deficiency
- Factor V Leiden mutation
- Prothrombin gene mutation
- Antiphospholipid antibodies
- Methylenetetrahydrofolate reductase mutation
- Prothrombotic medications (L-asparaginase, OCP)
- Maternal conditions
- Preeclampsia
- Diabetes
- Cocaine abuse
- Placental thrombosis
- Placental abruption
- Twin-twin transfusion
- Amnionitis
- Arteriopathy (17%-53% )
- Moyamoya disease
- Transient Cerebral Arteriopathy (TCA): Idiopathic, nonprogressive focal or segmental, unilateral stenosis of the distal ICA or proximal MCA/ACA resulting in a lenticulostriate infarction. 20-30% of stroke due to arteriopathies are caused by TCA (other terms focal cerebral arteriopathy (FCA), post-varicella angiopathy (PVA) or non-progressive primary angiitis of the central nervous system (PACNS)
- Progressive PACNS: Approx. 6% of arteriopathic strokes are due to PACNS which is rare, non-infectious, progressive arteriopathy isolated to the cerebral vessels without systemic involvement. It is characterized by bilateral distal and bilateral proximal cerebral artery narrowing
- Sickle cell disease(SCD): Approx. 10% children with SCD suffer stroke by age 20
- Cardiac diseases: Accounts for approx. 20-30% of stroke cases
- Congenital
- Acquired
- Arterial dissection: Approx. 7.5-20%
- Perinatal asphyxia is an association, may mimic stroke
- Neurofibromatosis (NF) type 1: Intracranial occlusive arterial disease is the neurovascular manifestation of NF type I in young patients; approx.6% of NF type 1 have vasculopathies
- Trauma to large arteries with dissection or occlusion
- Watershed strokes
- Comprises 10% of all ischemic strokes
- Occur in watershed or border-zone regions of brain supplied by major cerebral arteries
- Divided into two types: Cortical watersheds and internal watersheds (latter more common with distal ICA occlusion e.g. moyamoya)
Risk factors for perinatal stroke:
Maternal risk factors:
- History of infertility
- Chorioamnionitis
- Premature rupture of membrane
- Preeclampsia
- Other maternal and placental disorders
Neonatal risk factors:
- Cardiac disorders
- Coagulation disorders
- Infection, trauma, drugs
- Perinatal asphyxia
Epidemiology
Incidence/Occurrence:
- Birth-18 years: Approx. 6-8 per 100,000 children/year (30% neonatal stroke)
- Ischemic > Hemorrhagic
- Males > Females
- Possible ethnic/genetic factors; incidence higher in African Americans than Asian, Caucasians
- Mean age of childhood presentation is 4-6 years
- Morbidity
- 2/3 of neonatal and childhood stroke leads to motor deficit with lifetime disability and impairment
- 15% have chronic seizure disorders
- Mortality
- Childhood stroke:
- Approx. 10% following ischemic stroke and 25% following hemorrhagic stroke
- Mortality rate in first year is 5.3 per 100,000 children/year
- Pediatric stroke is one of the top ten causes of death in children
Incidence of Arterial Ischemic Stroke (AIS)
- Childhood: 2.4 per 100,000 children/year
- Neonatal/newborn: 20-30 per 100,000 newborns/year (~1/4000-5000 live births/year), although MRI detection of neonatal stroke suggests a higher incidence of ~1:2300 live births
- Perinatal AIS accounts for approximately 25-30 % of all AIS in children
Ref: Friedman, N Advances in Pediatrics. 2009, 56:271-299.
Incidence of Hemorrhagic Stroke
- Birth-18 years: 2.2 per 100,000 children/year
- Rate of hemorrhagic stroke for term infants is 6.7 per 100 000/year
Recurrence Rate
- Stroke recurrence risk is approximately 15-20%
- Recurrence rate for childhood AIS varies between 6-37% and is increased with arteriopathy
- AIS recurrence risk appears highest in the first 6 months after initial stroke
Pathophysiology
There is a low energy reserve in the brain; hence there is an absolute requirement for continuous oxygen and glucose. Any process that reduces cerebral perfusion leads to an oligemic state → ischemia → cell death via necrosis (infarction) or apoptosis (neuronal drop out).
Decreased cerebral perfusion triggers an “ischemic cascade” of events that mediate cell death. These changes include:
- Depletion of ATP
- Lactic acidosis
- Release of the excitatory amino acid glutamate → activation of glutamate receptors → membrane depolarization
- Loss of ionic homeostasis → changes in intracellular ion concentrations of sodium, potassium, and calcium
- Free radical (nitric oxide, superoxide) formation
- Membrane failure → cellular edema
- Activation of proteolytic enzymes and caspases
- Inflammatory cascade
- Ultimately leading to necrotic or apoptotic cell death
Clinical Presentation
Perinatal strokes:
In contrast to adult strokes, perinatal strokes may be asymptomatic initially, and deficits may only become apparent later on (PPIS see above). While it is uncommon to find focal deficits in acute newborn stroke, arterial and venous strokes often present with focal seizures, usually involving one limb.
Stroke may also be suspected in infants with
- Encephalopathy
- Asphyxiated appearance
- Signs of acute intercurrent illness
Caveat: Children with neurological symptoms present early as compared to adults (25% within 6 hours of onset). However, stroke is often not considered in the initial differential diagnosis in children, leading to delays in brain imaging; average delay in children is nearly 8 hours.
Ref: Bowers KJ et al. Journal of Child Neurology, 2011; 26: 1074-1100.
Signs/Symptoms in infants:
- Focal seizures (e.g. involving one limb)
- Perinatal distress, particularly in full-term neonates
- Problems feeding, breathing (gasping)
- Sudden hemi-body paresis or paralysis
- For those with delayed diagnosis (PPIS)
- Early preference for use of one hand over the other (note: hand dominance is not normal until 12 months of age)
- Delays in early milestones – rolling, sitting, pulling to stand, crawling, and walking
- Hemiplegic cerebral palsy
Signs/symptoms in children older than 1 year:
- Recurrent focal seizures: >50% cases may present in this fashion
- Sudden onset of any of the following:
- Hemi-body paresis or paralysis
- Neglect (hemibody, hemispatial)
- Difficulty with speech, swallowing
- Memory difficulty and learning disabilities; schoolwork may be affected
- Eye movement abnormalities
- Visual impairment; partial or complete blindness, diplopia, blurred vision
- Incoordination and impaired balance
- Horner’s syndrome (may signify carotid dissection or brainstem stroke)
- Severe headache
- Nausea and/or vomiting
Differential Diagnosis
Structural lesions:
- Brain tumors
- Intracranial abscess
Infectious processes:
- Meningoencephalitis
- Bilateral meningitis
- Post-infectious cerebellitis
- Botulinum
Demyelinating conditions:
- Acute disseminated encephalomyelitis (ADEM)
- Multiple sclerosis
- Transverse myelitis
Conditions with vasospasm:
- Reversible posterior leukoencephalopathy syndrome
- Complicated/hemiplegic migraine
Other:
- Postictal state (Todd’s paralysis-transient unilateral paresis/plegia)
- Intoxication
- Traumatic brain injury
Investigation and Workup
Neuro-imaging:
The optimal imaging study is dependent on the child’s clinical stability.
- Cranial ultrasound:
- Safe and quick
- Requires an open anterior fontanel
- Low sensitivity in detecting AIS and superficial lesions misses at least 33% of AIS
- Computed tomography (CT) scan (unenhanced):
- Relatively quick
- Useful for detecting hemorrhages, abscesses, and tumors
- Low yield for AIS in initial 24 hours
- Unable to assess venous thrombosis unless CT venogram
- CT-angiogram (CTA):
- Detection of stenosis, occlusions, dissections
- CT-venogram (CTV):
- Detection of cerebral sinovenous thrombosis (CSVT)
- Magnetic resonance imaging (MRI):
- Very high yield for detecting AIS, differentiating structural and demyelination lesions
- Also reliably detect hemorrhages
- First study of choice for suspected childhood AIS when feasible
- Magnetic resonance angiography (MRA):
- Detection of stenosis, occlusions, dissections, vascular malformations
- Magnetic resonance venography (MRV):
- Detection of cerebral sinovenous thrombosis (CSVT)
- Transcranial Doppler (TCD):
- Assessment of large vessel occlusive disease
- Possible assessment of neonatal cerebral sinovenous thrombosis CSVT
- Catheter angiography:
- Less commonly used
- Used in certain conditions such as vascular malformations, or vasculitis
NOTE: MRI/MRA/MRV modalities provide the highest yield and are preferred.
Other procedures and investigations are guided by suspicion of underlying pathology.
Suspected cardiac source of embolism:
- Transthoracic echocardiogram (TTE) or Transesophageal echocardiogram (TEE) for older children e.g. >7 years:
- Structural cardiac defects
- Thrombus
- Valve abnormalities (vegetations)
- Cardiac tumors
Infectious workup (suspected sepsis/meningitis):
- Blood and urine cultures
- Chest x-ray
- Lumbar puncture (also used to rule/out subarachnoid hemorrhage)
Hypercoagulability:
May require a full battery of investigations that can include
- PTT, INR, D-Dimer
- Protein C, protein S or antithrombin deficiencies
- Factor V Leiden gene mutation
- Anticardiolipin antibodies/Lupus anticoagulant
- Dilute Russell viper venom time
- Prothrombin gene mutations
- Beta2-glycoprotein I antibodies (IgG and IgM)
- Homocysteine
- Elevated lipoprotein(a)
- Factor VIII activity
Vasculitis:
- CTA, MRA, conventional angiography
- Antinuclear antibodies (ANAs)
- Herpes virus serology (varicella, HVS-1 & 2, EBV, and cytomegalovirus)
- Cerebrospinal fluid (CSF) analysis
Genetic/Metabolic: MELAS (Mitochondrial Encephalopathy, Lactic Acidosis, and Stroke)
- Mimics vaso-occlusive arterial stroke though regional metabolic infarction
- Maternally inherited multisystem disorder that may include stroke
- MRI may detect stroke overlapping vascular territories
- Elevation in serum or CSF lactate
- Muscle biopsy used to detect red ragged fibers
- Gene analysis for mitochondrial mutation (if required as diagnosis can usually be achieved prior to this)
Treatment
- Age (neonatal, older)
- Type of stroke (ischemic or hemorrhagic)
- Underlying cause(s)
- Acuity of the presentation (acute vs. chronic)
General supportive and neuroprotective measures:
Critical in preserving penumbra/limiting size of infarct
- Monitor vital signs/neuro vitals (in children) and maintain blood pressure at high range of normal for age
- Assess for changes, improvement or deterioration in symptoms
- Ensure adequate oxygenation; ventilatory support if required
- Control fever
- Treat dehydration (IV normal saline)
- Address anemia and platelet deficiency if present
- Monitor and correct metabolic disturbances:
- Acidosis
- Hypoglycemia
- Hypocalcemia
- Electrolyte disorders
- Antibiotic is used empirically if infection suspected (until results of culture available)
- Seizures-use anticonvulsants aggressively as required to control seizures (consider EEG monitoring if altered LOC)
- Cardiac monitoring to assess for arrhythmias
- Prevent venous thromboembolism by using pneumatic compression
ACUTE MANAGEMENT:
Acute Ischemic Stroke (AIS):
There is no data to support the use of thrombolytic therapy (tPA) in the pediatric age group, and is not recommended (outside the use of a clinical trial).
Neonates:
- Antithrombotic therapy is generally not indicated. Possible exceptions to use of LMWH includes:
- Detection of cardiac clot or complex congenital heart disease
- Multiple cerebral or systemic emboli
- Evidence of severe thrombophilic disorder
Childhood:
- Endovascular stent or clot retrieval treatment should not be attempted in infants and children with acute arterial ischemic stroke until age-specific safety data are available and until there is clear randomized controlled trial evidence of efficacy in adult ischemic stroke
- Anticoagulant therapy with heparin/LMWH may be initiated (within 7 days) for ~5-7 days, provided there are no contraindications
- Alternatively, antiplatelet agents (aspirin) can be used
- Following initial anticoagulant therapy patients may be further treated with either continued anticoagulant therapy or antiplatelet agents for secondary stroke prevention depending on the underlying cause (see secondary prevention below)
- Hemicraniectomy for malignant MCA infarction can be life-saving and should be initiated early for best outcomes (ideally prior to pupillary dilatation)
Cerebral Sinovenous Thrombosis (CSVT):
In general anticoagulant therapy indicated in both neonates and children, however, if significant cerebral hemorrhage identified, antithrombotics may be withheld initially unless clot extension evident by repeat neuroimaging (CTV or MRV).
Neonates:
- Anticoagulants should be considered for use in neonates with CSVT
- Treatment options include LMWH or UFH for minimum of 6 weeks and no longer than 3 months. Venous imaging is performed at 6 weeks and if full recanalization is seen, anticoagulation can be discontinued
Ref: (i) Saposnkik G. et al. Stroke. 2011;42:1158-1192
(ii) Moharir MD, Shroff M, Pontigon A-M et al. J Child Neurol. 2011; 26:1137-44
- Option: Monitor for clot propagation with venous imaging within 5-7 days if not anticoagulated (propagation present in 25-33% if not treated)
- LMWH 1.5 mg/kg/12 hr for 6-12 weeks
- Reassess thrombus with CTV or MRV at 6 wks. If fully recanalized then discontinue LMWH; if thrombus present continue for another 4-6 weeks
Childhood:
- LMWH 1 mg/kg/12h or coumadin (INR 2-3) for 3 months
- Reassess thrombus with CTV or MRV; if full recanalized then discontinue LMWH; if thrombus present continue for another 3-6 months
Hemorrhagic Stroke:
General supportive measures:
- Control hypertension gently
- Correct anemia/thrombocytopenia
- Control intracranial pressure
- Replace coagulation factor deficiencies if present
Neonates:
- Vitamin K administration for neonates, born to mothers who were on certain medications (i.e. barbiturates, phenytoin etc.) or who have vitamin K deficiency
- Ventricular drainage ±shunting for hydrocephalus
Childhood:
As with adults, brain hemorrhage in older children presents with acute headache, vomiting, and rapid deterioration of neurological function.
Management according to etiology
Intracranial aneurysms in children:
- Both microsurgical and endovascular techniques can be used for intracranial aneurysm repair
Vascular malformations in children: Congenital vascular anomalies in children include
- AVMs including in neonates vein of Galen malformations
- Endovascular embolization, microsurgical obliteration, and stereotactic radiotherapy can be used
- Dural fistula
- Endovascular embolization can be used
- Cavernous malformations/angiomas
- Low risk of disabling intracranial hemorrhage but may underlie epilepsy. May be treated conservatively (anticonvulsants) although microsurgery and stereotactic radiosurgery are potential options for some patients
- Venous angiomas/capillary telangiectasias
- Venous angiomas and capillary telangiectasias present a low risk of disabling intracranial hemorrhage and may be treated conservatively. However, microsurgery and stereotactic radiosurgery are potential options in symptomatic patients
Coagulation defects:
- Thrombocytopenia:
- Avoid antiplatelet agents, and avoid situations which may lead to head trauma
- Spontaneous bleeds are rare with platelet count >20,000 mm3, in the absence of trauma
- Overzealous infusion of platelets should be avoided to prevent development of allo-antibodies that may further decrease the platelet count
- Coagulation factor(s) deficiencies
- May require factor replacement therapy
Control of raised intracranial pressure:
- Surgical evacuation of hematoma may be employed to reduce elevated intracranial pressure. No data is available to show beneficial outcome
- Hydrocephalus may require ventricular drainage ±shunting
CHRONIC MANAGEMENT:
Rehabilitation:
- May be beneficial in children with residual deficits
Preventive therapy (ischemic stroke):
- Long-term use of prophylactic antithrombotic therapy (e.g. LMWH) or ASA is indicated in selected neonates (severe prothrombotic conditions and/or congenital cardiac defects) and in all children with AIS in whom either long-term ASA or anticoagulant therapy may be used depending on the underlying cause
- Children with sickle cell disease (SCD) and AIS may require regular exchange transfusions to reduce sickle hemoglobin to 30% total hemoglobin. For those intolerant to transfusions, hydroxyurea may be considered
- Children with moyamoya may benefit from indirect revascularization procedures (e.g.Encephalo-Dural-Arterio- Synangiosis, EDAS)
- Children with bilateral progressive vasculitis should be managed by a rheumatologist for long-term surveillance and anti-inflammatory treatments
Preventive therapy (hemorrhagic stroke):
- ICH caused by bleeding disorders may require prophylactic replacement coagulation factors
- Monitor and treat anemia
- Replace vitamin K if deficient
- Vitamin B and folate supplementation for hyperhomocysteinemia
Summary of management of neonatal stroke
Summary of management of childhood stroke
ROLE OF ANTITHROMBOTIC THERAPY IN CHILDREN WITH AIS:
Anticoagulation
- UHF: Employed in scenarios where quick reversal may be required. No bolus used
- LMWH:
- Easily administered and less monitoring
- Used for short and long-term anticoagulation
- Anti-XA activity; typically 0.5-1.0 for enoxaparin
- Warfarin: INR typically 2.0-3.0
- Used when long-term anticoagulation required, although LMWH is an alternative
- Dose adjustment is more difficult in breastfed infants due to low vitamin K levels in breast milk
- Considered for long-term anticoagulation with
- Risk of recurrent cardiac embolism
- Persistent severe hypercoagulable states
- CVST
Antiplatelet
Long-term secondary prophylaxis for TIA and AIS
- ASA 1-3 mg/kg/day
- Dipyridamole
- Clopidogrel
OVERALL PREDICTORS OF POOR OUTCOME OF STROKE IN CHILDREN
- Young age
- Altered consciousness at presentation
- Intractable intracranial hypertension
- Fever at presentation
- Middle cerebral artery territory stroke volume greater than 10% of the intracranial volume
- Right middle cerebral artery territory infarction
- Bilateral ischemia
- Combined cortical and basal ganglia involvement
- Presence of systemic illness
- Arteriopathy
OUTCOME
- 25% of children die after a hemorrhagic and 5% after ischemic stroke
- Mortality is higher for hemorrhagic than for ischemic
- 60-80% of surviving children have neurological complications, most commonly hemiparesis
- Neurological outcome appears to be better for those with hemorrhage, CVST, and posterior circulation stroke
- Age-appropriate rehabilitation, support groups for caregivers and therapy programs are indicated for children after a stroke
- Psychological assessment to document cognitive and language deficits is useful for planning therapy and educational programs after a child’s stroke
[/cq_vc_tab_item][cq_vc_tab_item tabtitle=”Medication Dose”]MEDICATIONS
- Heparin
- Low-molecular-weight heparin (LMWHs)
- Enoxaparin
- Dalteparin
- Tinzaparin
- Reviparin (not available in Canada and USA)
- Oral anticoagulants (OAC)
- Warfarin
Mechanism:
- Inhibits thrombus formation and prevent extension of existing thrombi
- No direct lytic effect on established thrombi
- Prolongs aPTT, PT and INR
Dose:
Heparin
Initial bolus dose
- Usually no bolus given or if necessary give 75 to 100 units/kg over 10 minutes
Initial maintenance dose
- Age <1 years: 28 units/kg/hr
- Age >1 years: 20 units/kg/hr
- Older children: 18 units/kg/hr
Note: Adjust infusion rate to achieve and maintain a target aPTT of 60 to 85 seconds (anti-Xa activity 0.3-0.7units/ml)
Protocol for Systemic Heparin Administration and Adjustment in Children
Treatment of Heparin-Induced Bleeding
LMWH
Note: Dosage adjustment should be based on measurement of anti-FXa levels 2 to 6 hours after subcutaneous injection (target 0.5-1.0 U/ml).
Enoxaparin:
- Age <2 months: Initial treatment dose 1.5 mg/kg/12hr; initial prophylactic dose 0.75 mg/kg/12hr
- Age >2 months and ≤18 years: Initial treatment dose 1.0 mg/kg/12hr; initial prophylactic dose 0.5 mg/kg/12hr
- Enoxaparin Pediatric Dosage Titration
Dalteparin: (not an approved use in Canada)
- Initial treatment dose 129 ±43U/kg/24 hr; initial prophylactic dose 92 ±52 U/kg/24hr
Tinzaparin:
Reviparin: (not available in Canada and USA)
- Weight <5 kg: Initial treatment dose 150 mg/kg/12hr; initial prophylactic dose 50 mg/kg/12hr
- Weight >5 kg: Initial treatment dose 100 mg/kg/hr; initial prophylactic dose 30 mg/kg/12hr
Summary of LMWH use in Children
Treatment of LMWH-Induced Bleeding:
- Protamine sulfate required is dependent on the dose of LMWH used Repeat doses of protamine
Warfarin
- Aspirin
- Clopidogrel
- Dipyridamole
Mechanisms:
Aspirin
- Works through cyclooxygenase pathway (COX 1-2 )
- Inhibits platelet aggregation
- Antipyretic, anti-inflammatory and analgesic action
- Antiplatelet effects last ~7-10 days
Clopidogrel
- Binds to adenosine diphosphate (ADP) → impairs activation of receptor complex → inhibits platelet aggregation
- Antiplatelet effects last ~7-10 days
Dipyridamole
- Works through adenosine, adenine nucleotides, and cyclic AMP → inhibits adenosine deaminase and phosphodiesterase → Inhibits platelet aggregation
Dose:
Aspirin
- 1-5 mg/kg/day once daily (ACCP)
- 3 to 5 mg/kg per day, with the dose reduced to 1 to 3 mg/kg in response to gastric distress or prolonged epistaxis (AHA)
Note: To reduce the risk of Reye’s syndrome after influenza and varicella, it is reasonable to give an annual influenza immunization, or verify the status of varicella vaccination, and consider halting the use of aspirin during suspected influenza or varicella infections.
(Roach ES et al AHA Stroke Council and the Council on Cardiovascular Disease in the Young Stroke 2008; 39: 2644-2691
Clopidogrel:
- ~1 mg/kg/day; max dose 75 mg OD
Dipyridamole:
- 2-5 mg/kg/day
[/cq_vc_tab_item][/cq_vc_tabs]
Physician Resources
1. Tips for Patient Care
- Ensure parents and patient (whenever possible) are well informed about disease and its outcomes
- The patient (whenever possible) and family should be encouraged to express their main concerns about reintegration to the home, community and school environments
- Anemia, thrombocytopenia and factor deficiencies should be monitored and corrected
- Clinical or laboratory signs of inflammation are not always present in all individuals with vasculitis
- Pregnancy is considered a stroke risk factor in teenaged girls
- Signs and symptoms of stroke are more diffuse and less focal in neonates and infants as compared to older children
- New onset neurological symptoms or signs in children with sickle cell disease should be evaluated as potentially being due to stroke
- MRI is the most sensitive imaging technique for identifying ischemic brain lesions
- Sensory function in young children is difficult to assess
Medications:
- Consider concurrent risk factors and disease states with the prescribed therapy; example aspirin may worsen the symptoms of asthma
- It is reasonable to reduce aspirin dosage by 50% during influenza and varicella infections (to reduce risk of Reye’s syndrome)
- Large doses of vitamin K may be needed to correct factor deficiencies induced by maternal medications
- tPA generally is not recommended for children with AIS
- Anticoagulant therapy usually is not recommended in individuals with bacterial endocarditis
Social and Stress factors:
- Direct parents and patients to appropriate support services/counseling to help alleviate psychological stress, whenever its possible
Counseling
- Ensure family and/or caregivers as well as patient (whenever possible) are well informed about disease and potential triggers
- Thrombophilia screening may be offered to
- Family members of children with ischemic stroke or CVST and known thrombophilic defects
- Mothers of children with ischemic stroke that occurred before, during, or immediately after birth even if thrombophilia screening in the neonate is negative
Physical activity:
- Rehabilitation and physical therapy are among important measures to improve neurological outcome in individuals with perinatal stroke
- Include family or social support in lifestyle modification
Alerts
- Venous thrombosis and early acute ischemic stroke(AIS) is usually missed with CT
- Approximately 10% of seizures in term neonates are due to stroke
- Approximately 30% of intraventricular haemorrhage in term neonates is due to CSVT
- Stroke/ICH are major complications of sickle cell disease (Ref: Wu, Ferriero et al)
- Intracranial infections can cause vasculitis and imaging should be considered
Expected outcome
- Outcome of children with perinatal stroke ranges from normal to hemiparesis (subtle, moderate or severe) and mild neurocognitive delays
- Most survivors with neonatal AIS and CVST learn to walk independently before 2 years old
- Neonatal encephalopathy is predictive of poor outcome after AIS
2. Scales and Tables
- Protocol for Systemic Heparin Administration and Adjustment in Children
- Treatment of heparin-induced bleeding
- Warfarin Anticoagulation Protocol for Children
- Treatment of VKA-induced bleeding
- Comparison of AACP and AHA recommendation for management of childhood AIS
- Comparison of AACP and AHA recommendation for maintenance therapy following childhood AIS
References
Core Resources:
- Bowers KJ; DeVeber GA; Ferriero DM et al Cerebrovascular disease in children: Recent advances in diagnosis and management Journal of Child Neurology 2011;26(9):1074-1100
- Patty Lindsay et al. last publication of Canadian BPG’s which included Ped Stroke Guidelines consistent with this article
- Compendium of Pharmaceuticals and Specialties (CPS). Canadian Pharmacist Association. Toronto: Webcom Inc. 2012
- Lynch JK, Hirtz DG, deVeber G, Nelson KB. Report of the National Institute of Neurological Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics. 2002; 109: 116-23
- deVeber G, Andrew M, Adams C et al. Cerebral sinovenous thrombosis in children. Canadian Pediatric Ischemic Stroke Study Group. N Engl J Med. 2001;345:417-23
- Friedman, N Pediatric Stroke: Past, Present and Future. Advances in Pediatrics. 2009, 56:271-299
- Katzung BG, Masters SB, Trevor AJ, eds. Basic and Clinical Pharmacology. 11th ed. New York: McGraw-Hill; 2009
- Lindsay P, Bayley M, Hellings C, et al. Canadian Best Practice recommendations for stroke care.CMAJ.2008; 179: S1-S25
- Monagle P, Chalmers E, Chan A, et al. Antithrombotic Therapy in Neonates and Children American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8thEdition) CHEST 2008; 133: 887S-968S
- Monagle P, Chan A, Goldenberg NA et al. Antithrombotic Therapy in Neonates and Children: : Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines Chest 2012;141;e737S-e801S
- Roach ES, Golomb MR, Adams R, et al. Management of Stroke in Infants and Children A Scientific Statement From a Special Writing Group of the American Heart Association Stroke Council and the Council on Cardiovascular Disease in the Young Stroke 2008; 39: 2644-2691
- Saposnkik G. Barinagarrementeria F, Brown RD et al. Diagnosis and Management of Cerebral Venous Thrombosis; Stroke. 2011;42:1158-1192
- Skidmore-Roth L. ed. Mosby’s drug guide for nurses. 9th ed. St. Louis: Elsevier-Mosby; 2011
- Skidmore-Roth L, ed. Mosby’s nursing drug reference. 24th ed. St. Louis: Elsevier-Mosby; 2011
- Wu, Ferriero et al
- Online resources:
- The Heart and Stroke Foundation
- National Institute of Neurological Disorders and Stroke
Online Pharmacological Resources:
- e-Therapeutics
- Lexicomp
- RxList
- Epocrates
Journals/Clinical Trials:
- Agrawal N, Johnston SC, Wu YW. Et al. Imaging Data Reveal a Higher Pediatric Stroke Incidence Than Prior US Estimates. Stroke. 2009; 40: 3415-3421
- Alexiou GA, Mpairamidis E, Sfakianos G, Prodromou N. Surgical management of brain cavernomas in children. Pediatr Neurosurg. 2009;45:375-8
- Bensler SM, Silverman E, Aviv RI; Primary Central Nervous System Vasculitis in Children; Arthritis & Rheumatism 2006, 54,1291-1297
- Goldenberg NA, Bernard TJ, Fullerton HJ, Gordon A, deVeber G; International Pediatric Stroke Study Group. Antithrombotic treatments, outcomes, and prognostic factors in acute childhood-onset arterial ischaemic stroke: a multicentre, observational, cohort study. Lancet Neurol. 2009;8:1120-7
- Jordan LC, Hillis AE. Hemorrhagic Stroke in Children Pediatr Neurol. 2007 February; 36: 73-80.
- Kincaid PK, Duckwiler GR, Gobin YP, Viñuela F. Dural arteriovenous fistula in children: endovascular treatment and outcomes in seven cases. AJNR Am J Neuroradiol. 2001;22:1217-25
- Moharir MD, Shroff M, Pontigon A-M et al. A prospective outcome study of neonatal cerebral sinovenous thrombosis J Child Neurol. 2011; 26:1137-44
- Rafay MF, Pontigon A-M, Chiang J et al. Delay to Diagnosis in Acute Pediatric Arterial Ischemic Stroke Stroke. 2009; 40:58-64
- Schechter T, Kirton A, Laughlin S et al. Safety of anticoagulants in children with arterial ischemic stroke Blood. 2012; 119: 949-956
