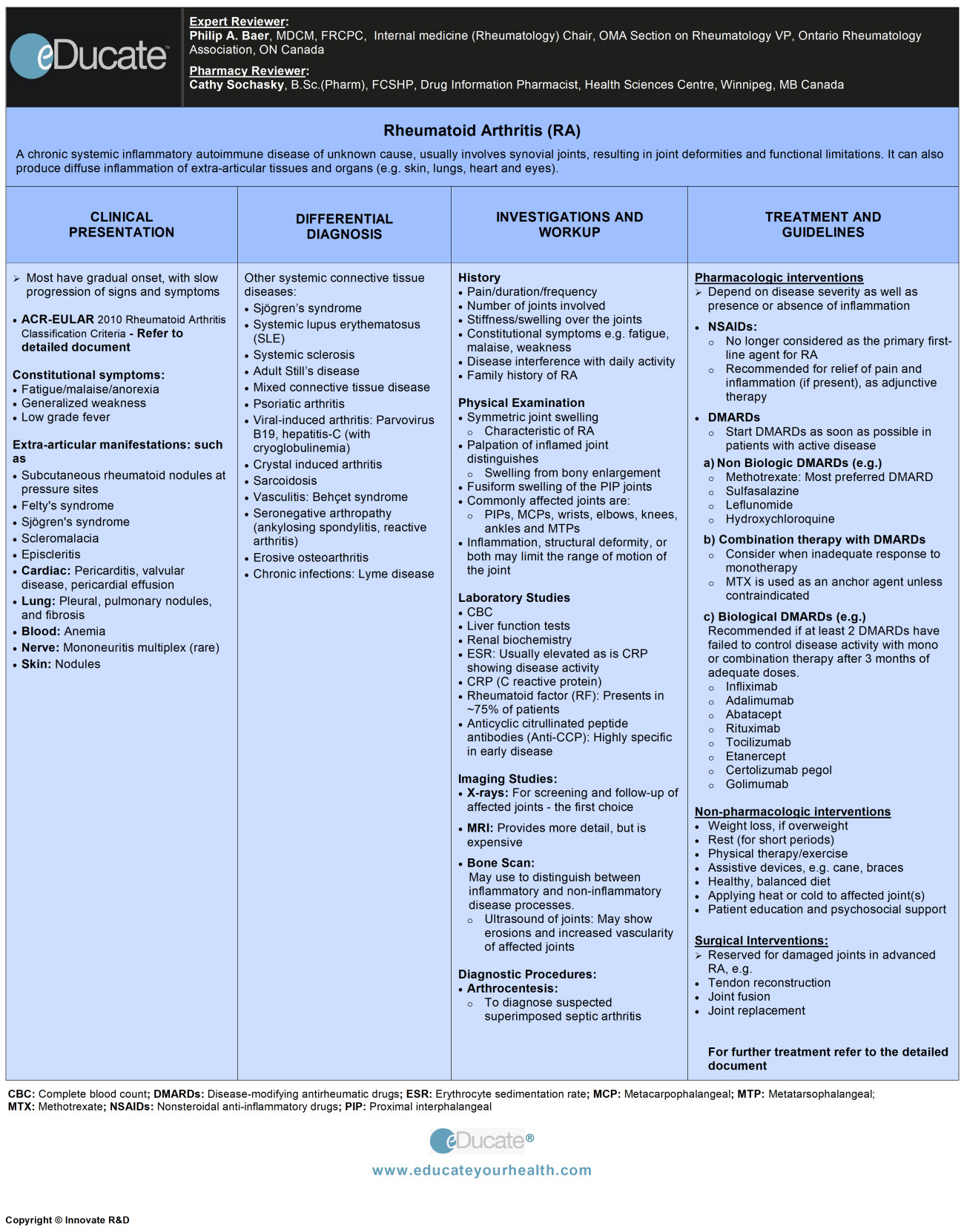
Rheumatoid Arthritis (RA)
Philip A. Baer, MDCM, FRCPC, Internal medicine (Rheumatology), Chair, OMA Section on Rheumatology, VP, Ontario Rheumatology Association, ON Canada
PHARMACY REVIEWER
Cathy Sochasky, B.Sc.(Pharm), FCSHP, Drug Information Pharmacist, Health Sciences Centre, Winnipeg, MB Canada
Definition
A chronic systemic inflammatory autoimmune disease of unknown cause, involving synovial joints, resulting in joint deformities and functional limitations if not adequately controlled. The disease can also produce systemic inflammation and involve extra-articular structures and organs (e.g. skin, lungs, heart, and eyes).
Etiology
The precise cause is unknown. Various factors can contribute and include:
- Environmental: Smoking induces formation of citrullinated peptides in susceptible individuals leading to formation of antibodies to Cyclic Citrullinated Peptides (anti-CCP) and Rheumatoid Factors (RF) triggering auto-immunity which underlies Rheumatoid Arthritis (RA)
- Infection: Various pathogens (e.g. viruses and mycoplasma) induce inflammation and triggers autoimmunity in a susceptible host leading to RA. None of the potential pathogens appear to be RA specific, and no specific pathogen is definitely linked to RA
- Hormonal: Female predominance, ~3 fold >males. Estrogen may play a role in RA development by facilitating immune system activation
- Genes: Play role in susceptibility
- May account for ~50% risk of developing RA
- May impact disease severity
- Class II MHC genes (containing a specific 5-amino acid sequence in the hypervariable region of HLA-DR4, called the SHARED EPITOPE), have been implicated
- Immunologic Factors:
- Complex process involving T and B lymphocytes, antigen presenting cells (macrophages and dendritic cells) and cytokines. The latter may have pleiotropic actions and multiple targets and may exhibit both pro-inflammatory and anti-inflammatory actions. Controlling the balance between competing cytokines is considered as an important therapeutic goal. Key pro-inflammatory cytokines are TNF, IL-1, and IL-6
- T cells activate macrophages/synovial fibroblasts which produce proinflammatory cytokines leading to initiation of RA
- B cells produce autoantibodies/cytokines eventually contributing to pathological injury
- The synovial membrane is ultimately transformed into pannus tissue with activated synovial cells and osteoclasts eroding cartilage and bone.
Epidemiology
- Rheumatoid arthritis (RA) affects about 1% of the population
- Women are affected ~3 times more than men
- Onset may be at any age, most often appears between the ages of 35 and 50 years
Pathophysiology
Pathogenesis is not completely understood, but may include:
- Autoimmune reaction triggered by infection/trauma → synovial hypertrophy → joint inflammation (synovitis)
- Subsequent uncontrolled inflammation → cartilage and bone destruction
- Increased immune activity in genetically susceptible individuals, facilitate the disease
- Immune activity involves:
- T cells, phagocytes, fibroblasts, osteoclasts, and neutrophils
- B cells produce characteristic autoantibodies (i.e. rheumatoid factors [RFs] and anti-CCP)
- Aberrant production of numerous cytokines, inflammatory mediators (e.g. TNF & IL-1), and growth factors ensues
- Inflammation, excessive proliferation of synovium, formation of granulation tissue (i.e. pannus) promotes tissue destruction
Clinical Presentation
- Some experience a gradual onset, with slow progression of signs and symptoms, however many patients may present with progressive joint pain and stiffness, functional limitation and radiographic damage
- RA is also associated with significant morbidity and increased mortality, primarily related to cardiovascular disease and infections
- RA has been redefined as of 2010 by ACR/EULAR, based on the following criteria
Signs and symptoms include:
Gradual onset, slowly progressive joint pain
Morning stiffness ( may last 45 minutes or longer)
Functional limitation
Constitutional symptomsFatigue/malaise
Anorexia
Generalized weakness
Low-grade fever
Boutonniere deformity: PIP joint is flexed and DIP joint hyperextended
Swan-neck deformity: DIP joint is flexed and the PIP joint is hyperextended
Baker’s cyst: The synovial sac of knee joint produces a bulge into the popliteal space and forms a cystic swelling, which can rupture and cause pain
Extra-articular manifestations:
Less common with better therapy
Subcutaneous rheumatoid nodules at sites of pressure and chronic irritation
Damage to the ligaments and tendons
Vasculitis causing leg ulcers
Lymphadenopathy
Felty’s syndrome (splenomegaly and leukopenia)
Sjögren’s syndrome (dry eyes, mouth, skin etc.)
Scleromalacia
Episcleritis
Involvement of the cervical spine can cause atlantoaxial subluxation and spinal cord compression
Cardiac: Pericarditis, valvular disease, pericardial effusion (usually asymptomatic)
Lung: Pleural effusion with very low glucose level, pulmonary nodules, infiltrates and fibrosis
Blood: Anemia with normal MCV
Nerve: Mononeuritis multiplex
Skin: Nodules at pressure points
Differential Diagnosis
Other systemic connective tissue diseases:
- Sjögren’s syndrome
- SLE
- Systemic sclerosis
- Adult Still’s disease
- Mixed connective tissue disease
- Psoriatic arthritis
- Viral-induced arthritis: Parvovirus B19, hepatitis-C (with cryoglobulinemia)
- Crystal-induced arthritis
- Sarcoidosis
- Vasculitis: Behçet syndrome
- Seronegative arthropathy (ankylosing spondylitis, reactive arthritis)
- Erosive osteoarthritis
- Chronic infections: Lyme disease
Investigation and Workup
History: Detailed history should include:
- History of joint pain
- Duration
- Number of joints involved
- Frequency
- History of stiffness/swelling over the joints
- History of constitutional symptoms e.g. fatigue, malaise, weakness
- Inquiries about quality of life
- Inquiries if disease interferes with daily activities
- Family history of RA
Note: Multidimensional Health Assessment Questionnaire (mdHAQ) forms are very helpful in documenting all aspects of the history. See section: 11 for Physician Resources.
Physical Examination:
Assess joint inflammation and structural deformity. Commonly affected joints which are easily detected on examination are proximal interphalangeal (PIP), metacarpophalangeal (MCP), wrists, elbows, knees, ankles and metatarsophalangeal (MTP).
- Symmetric joint swelling is characteristic of RA
- Palpation of joint can distinguish swelling from bony enlargement
- Fusiform swelling of the PIP joints of the hands is a common early finding
- Redness is not a prominent feature for RA, but can occur
- Pain occurring with both active and passive movement is a hallmark of joint inflammation
- Inflammation, structural deformity, or both may limit the range of motion of the joint
- Persistent tenosynovitis and synovitis may lead to the formation of synovial cysts, and to displaced or ruptured tendons
Laboratory Tests:
The presence of prognostic features should be assessed and considered when making treatment decisions; they are also used to monitor disease activity and medication effects:
- Complete blood count (CBC), liver function tests (LFTs)
- Urea, creatinine
- ESR/CRP: Usually elevated
- CRP (C reactive protein)
- Rheumatoid factor (RF)
- Present in ~75% of patients
- Reflects disease activity to some extent
- ACCP (anti-cyclic citrullinated peptide antibodies): Highly specific in early disease
Screening before starting biologic therapy:
- Hepatitis B and C (and HIV in high-risk patients)
- Screening for latent tuberculosis is recommended prior to anti-TNF, abatacept, and tocilizumab
- Baseline antinuclear antibody (ANA) may be considered
Imaging:
X-rays: First choice for screening of affected joints
- Images of hands/feet may be done as frequently as every 6-12 months in patients with recent-onset disease, and at longer intervals in those with established disease
MRI:
- May provide more detail of erosions, synovitis and bone edema, but is expensive and not widely available
Ultrasound:
- May help in visualizing effusions, erosions, and synovitis by grey scale and power Doppler assessments
- Helpful in assessing some joints (e.g. hip joints) in obese patients
Bone scan:
- Radionuclide scans distinguish between inflammatory and non-inflammatory processes
- Bone densitometry provides info regarding osteoporosis
Diagnostic Procedures:
Arthrocentesis:
- Needed to diagnose superimposed septic arthritis
- Considered if one joint is inflamed out of proportion to other joints.
Findings consistent with RA are:
- Yellowish-white fluid, turbid, poor viscosity
- WBC increased (3,500-50,000)
- Negative for crystals and on culture
Diagnostic criteria:
- Early intervention improves disease outcome and decreases joint damage
- The criteria are is aimed at assessing those patients with clinical synovitis or with synovitis not explained by any other cause. The number and types of joints (large and/or small) involved, serology for (rheumatoid factor) RF and/or (anti-cyclic citrullinated protein antibody) ACPA, acute phase reactants (CRP and ESR) and duration of symptoms are all taken into account when making the diagnosis of RA. Patients presenting with new symptoms as well as those with the existing erosive disease should be reassessed to determine if they fulfill the 2010 criteria for RA
- RA has been redefined as of 2010 by ACR/EULAR, based on the following criteria:
Treatment
- Nutrition
- Rest
- Physical measures
- Joint splinting
- Orthopedic or athletic shoes
- Exercises
- Heat and cold therapy
- Paraffin baths
- Massage
Surgical interventions:
- Tendon reconstruction, joint fusion, and joint replacement are potential treatment modalities to prevent disability in advanced RA
Follow-up recommendations
- Monitor patient regularly
- Address risk factors and evaluate for osteoporosis, major comorbidity that can result from the disease itself or corticosteroid use
- Cardiovascular disease is the number one cause of death. Evaluate and manage cardiovascular risk factors; low dose aspirin may be used as prophylactic therapy
Prognosis
- Poor prognostic findings:
- Persistent moderate to severe disease
- Positive anti-CCP antibody
- Inheritance of shared epitope
- Early or advanced age at disease onset
- 50% cannot function in primary job within 10 years of onset
[/cq_vc_tab_item][cq_vc_tab_item tabtitle=”Pharmacologic therapy”]Goal:
- Relieve signs and symptoms
- Inhibit disease progression by early diagnosis and intervention
- Improve quality of life
- Achieve remission or at least low disease activity
Treatment involves:
- Pharmacological interventions
- Non-pharmacological interventions
- Surgical interventions
PHARMACOLOGICAL INTERVENTIONS:
- Patients are symptomatically treated as they present to the clinic. Previously undiagnosed patients with possible RA diagnosis should be given an urgent referral to the rheumatologist for definitive diagnosis and treatment
- Treatment approach has undergone major changes over the years:
- NSAIDs are beneficial only for relief of symptoms
- NSAIDs have no effect on disease progression
- Irreversible joint damage may occur in early disease
1. NSAIDs:
- Recommended for the relief of symptomatic pain and inflammation (if present)
- No longer used as the sole first-line agent for RA
2. Disease-modifying antirheumatic drugs (DMARDs):
Start DMARDs immediately if patient has active disease.
- Active RA patients require frequent monitoring (every 1-3 months)
- Patients with well-controlled disease may be monitored less frequently
- DMARD should be adjusted every 3-6 months, to achieve treatment goals
Ref: Bykerk V P et al. J Rheumatol 2011; 38:11
Types of DMARDs:
- Nonbiologic
- Biologic
Nonbiologic DMARDs
Preferable agents due to fast onset of action and less toxicity.
- Methotrexate (MTX): Most preferred of all the DMARDs
- 7.5-25 mg per week PO, plus folic acid 5mg PO daily to prevent from toxicity
- Although unlabeled route, subcutaneous route may be more effective and better tolerated, especially at doses greater than 15 mg/week. The dose should be escalated quickly to at least 15-20 mg/week in most patients
- Monitor CBC, renal, and liver function every 8-12 weeks
- Contraindicated in renal disease
- Most patients treated with MTX exhibit clinical and radiological improvement, a change in therapy should be considered in patients with radiographic progression despite adequate clinical response
- Sulfasalazine: 500 mg per day; max: 2-3 g per day; 3-month trial. Monitor CBC, liver enzymes every 8-12 weeks. Screen for G6PD deficiency
- Hydroxychloroquine: Dose is based on body weight not to exceed 400 mg per day; or 6.5 mg/kg per day in general. Used in milder forms or in combination with other DMARDs. Yearly ophthalmologic exam. Adjust dose in renal insufficiency
- Minocycline /Cyclosporin/Azathioprine: Can be considered as alternative options
- Leflunomide: 10-20 mg per day. Modifies T-cell function to decrease autoimmune activity and reduce structural damage. Side effects: GI disturbances, hair loss, hypertension, liver and lung toxicity; also potentially teratogenic
- Intramuscular gold /D-penicillamine: Rarely induce sustained remission. Have been largely replaced by more effective agents
Note: Glucocorticoids may be used for flare-ups while initiating or waiting for DMARDs to take effect. Lowest possible effective dose for a short time period is suggested
- Prednisone: 5-10mg/day is commonly used in patients to bridge the therapy in early RA. Once the disease is controlled; it should be weaned to the lowest possible dose, and then stopped to avoid adverse effects
- Combination therapy with DMARDs indicated if:
- Poor prognostic features
- Moderate to high disease activity in newly diagnosed patients
- Poor response to monotherapy
- MTX is used as an anchor agent.
Biologic DMARDs:
- Switch to biological agents if:
- At least 2 nonbiologic DMARDs (including MTX), have failed to control disease activity with mono or combination therapy after 3 months of targeted dose
- Agents include:
- Tumor necrosis factor inhibitors
- Etanercept
- Adalimumab
- Certolizumab pegol
- Golimumab
- Infliximab
- T cell costimulatory inhibitors
- Abatacept (ABAT)
- B lymphocyte depleting agent
- Rituximab (RTX)
- Interleukin 6 (IL-6) antagonist
- Tocilizumab (TCZ)
- IL-1receptor antagonist
- Anakinra
- Tumor necrosis factor inhibitors
No evidence to suggest that one is superior to the other, though anakinra appears to be the least effective agent, except for Adult Still’s disease variant of RA. The combination with MTX appears to be the most effective regimen for most biologics.
If a patient achieves sustained remission after discontinuation of NSAID and glucocorticoids, a reduction in traditional and biologic DMARD can be attempted.[/cq_vc_tab_item][cq_vc_tab_item tabtitle=”Medication Dose”]
TYPES
- Salicylates
- Acetic acid derivatives
- Diclofenac
- Etodolac
- Indomethacin
- Sulindac
- Enolic acid (Oxicam) derivatives
- Meloxicam
- Piroxicam
- Tenoxicam
- Napthylkanone derivatives
- Nabumetone
- Propionic acid derivatives (profens)
- Fenoprofen
- Flurbiprofen
- Ibuprofen
- Ketoprofen
- Naproxen
- Oxaprozin
- COX-2 inhibitors
- Celecoxib
NSAIDs Mechanism(s):
- Prostaglandins are common locally produced chemicals mediating pain, fever, and inflammation
- These drugs reversibly inhibit cyclooxygenase-1 and 2 (COX-1 and 2) enzymes
- This results in decreased formation of prostaglandin precursors
Acetylsalicylic acid: (additional mode of action)
- Irreversibly interferes with the production of thromboxane A2 within the platelet, thus inhibiting platelet aggregation
NSAIDs Doses:
Salicylates: (rarely used for RA therapy at present)
Aspirin
- 325-650 mg PO daily, every 4-6 hrs; Max. 4 g/day
Diflunisal
- 500-1000 mg/day PO in 2 divided doses
Renal impairment:
- CrCl<50 mL/minute: Decrease by 50% of normal dose
Acetic Acid Derivatives:
Diclofenac
- Immediate-release: 50 mg PO BID or TID; Max. 150 mg/day
- Slow-release: 75-100 mg PO once a day; Max. 150 mg/day
- Rectal suppository: 50-100 mg/day; Max. 100 mg/day or combined dose (rectal+oral) is 150 mg/day
Diclofenac/Misoprostol
- 50 mg/200 mcg and 75 mg/200 mcg tablets; Max. 150 mg Diclofenac/day
Etodolac
- Immediate-release: 200-300 mg PO BID or TID; Max. 1000 mg/day
Indomethacin
- 25-50 mg PO BID or TID; Max. 200 mg/day
- Extended-release: 75 mg PO BID or TID; Max. 150 mg/day
Sulindac
- 150-200 mg PO BID; Max. 400 mg/day
Enolic Acid (Oxicam) Derivatives:
Meloxicam
- 7.5 mg PO once daily; may increase to 15 mg daily; Max. 15 mg/day
Piroxicam
- 10-20 mg PO once daily; Max. 20 mg/day
Tenoxicam
- 10-20 mg PO once daily; usual dose 20 mg PO once daily
Napthylkanone Derivative:
Nabumetone
- 1000 mg PO once daily; may increase up to 2000 mg/day in 2 divided doses
Renal impairment:
- CrCl 30-49 mL/minute: 750 mg/day may increase up to 1500 mg/day
Propionic Acid Derivatives (profens):
Fenoprofen
- 300-600 mg PO TID or QID; Max. 3.2 g/day
Flurbiprofen
- 200-300 mg PO daily in 2,3 or 4 divided doses
Note: Do not administer more than 100 mg per single dose; Max 300 mg/day
Ibuprofen
- 200-800 mg PO TID or QID; Max. 3.2 g/day
Ketoprofen
- Regular release: 150-200 mg PO TID or QID; Max. 300 mg/day
- Extended release: 200 mg PO daily
- Renal impairment:
- Mild: 150 mg/day
- Severe (CrCl <25mL/min): 100 mg/day
Naproxen
- Regular release: 250-500 mg PO BID; may increase to 1.5 g/day
- Extended release: 750-1000 mg PO BID or once daily
- EC-Naproxen + IR-Esomeprazole: 375/20 mg or 500/20 mg PO BID
Oxaprozin
- 600-1200 mg PO daily; Max. 1800 mg or 26 mg/kg, whichever is less
COX-2 inhibitors:
Celecoxib
- Initial 100 mg PO BID may increase to 200 mg/day PO BID; Max. 200 mg/day
- Acetaminophen
Mechanism:
- Analgesic action: Inhibits the synthesis of prostaglandins in the central nervous system
- Antipyretic action: Inhibits the hypothalamic heat-regulating center
Dose:
Acetaminophen
- 325-650 mg PO every 4-6 hrs or 1000 mg PO TID or BID; Max. of 4 g/day and 1 g/dose
- Betamethasone
- Methylprednisolone acetate (most commonly used)
- Triamcinolone acetonide
- Triamcinolone hexacetonide
Mechanism:
- Decreases inflammation and the normal immune response through multiple mechanisms, also suppresses adrenal function at high dose, also has mineralocorticoid activity.
Doses:
Betamethasone (Intra-articular):
- Hip: 1-2 ml
- Knee, ankle, shoulder: 1 ml
- Elbow, wrist: 0.5-1 ml
- Metacarpophalangeal, sternoclavicular: 0.25-0.5 ml
Methylprednisolone (Intra-articular):
- Ankle, shoulder: 40 mg dose
- Hip: 80-160 mg
- Knee: 40-80 mg
- Elbow, wrist: 20-40 mg
- Small joint like MCP, PIP, DIP, SC joint: 10 mg
Triamcinolone acetonide (Intra-articular):
- Large joints: 5-40 mg
- Small joints: 2.5-10 mg
Triamcinolone hexacetonide (Intra-articular):
- Large joints: 10-20 mg
- Small joints: 2-6 mg
NONBIOLOGIC DMARDs
Antimetabolite/Antirheumatic Agent
- Methotrexate
Mechanism:
- It is a folate antagonist that inhibits DNA synthesis and cell reproduction
- Inhibits purine and thymidylic acid synthesis
- It also has immune modulator and anti-inflammatory actions
Dose:
Methotrexate
- Oral: Initiate at 7.5-15 mg/week PO given as a single dose or divided into 3 equal doses at 12 hour intervals for 3 doses along with folic acid; Max. 25 mg/week
- Subcutaneous: 7.5-25 mg once weekly
Anti-inflammatory (5-Aminosalicylic Acid Derivative)
- Sulfasalazine
Mechanism:
- Actual mechanism not determined
- Interferes with secretion by prostaglandin synthesis inhibition
Dose:
Sulfasalazine
Slow release formulation prescribed as follows:
- Week 1 = 500 g/day PO at bedtime daily
- Week 2 = 500 g/day PO BID
- Week 3 = 500 g/day PO in the morning and 1000 g PO at bedtime
- Week 4 and onwards: 1000 g/day PO BID
- Note: May increase to maximum 3 g/day, if response to 2 g/day is inadequate after 2 months of use
Antirheumatic Agent, Gold Compound
- Sodium aurothiomalate (rarely used in current management of RA)
Mechanism:
- Unknown, may decrease prostaglandin synthesis or may alter cellular mechanisms by inhibiting sulfhydryl systems
- It exhibits anti-inflammatory, antiarthritic and immunomodulating effects
Dose:
Sodium Aurothiomalate
- Intramuscular injections: 1st week: 10 mg IM, 2nd week: 25 mg IM, then 25 to 50 mg IM weekly for the next 20 weeks or until toxicity occur
- Maintenance: 50 mg IM tapered progressively to every 2 to 4 weeks according to clinical response and tolerance, and maintained indefinitely
Antirheumatic/Immunomodulator Agent
- Leflunomide
Mechanism:
- Inhibits pyrimidine synthesis via dihydroorotate dehydrogenase, resulting in antiproliferative and anti-inflammatory effects
Dose:
Leflunomide
- 20 mg PO daily; may decrease dose to 10 mg PO daily if 20 mg PO is not tolerated well
Anti-inflammatory/Antimalarial
- Hydroxychloroquine
Mechanism:
- Exact mode of action in controlling these diseases is unknown
- Antirheumatic/immunosuppressive effects: Inhibits rheumatoid factor, acute phase reactants, and many enzymes
Dose:
Hydroxychloroquine (rarely used now)
- Start 200-400 mg/day PO; do not exceed 6.5 mg/kg dose
- Note: Eye checkups for retinal toxicity required on an annual basis
- Azathioprine
Mechanism:
- Complete mechanism of immunosuppression is not fully known
- It antagonizes purine metabolism and may inhibit synthesis of DNA, RNA, and proteins
- May also interfere with cellular metabolism and inhibit mitosis
Dose:
Azathioprine
- Start 1 mg/kg/day PO daily or in 2 divided doses for 6-8 weeks; may increase by 0.5 mg/kg every 4 weeks, up to 2.5 mg/kg/day; for minimum of 12 weeks
- Maintenance dose: Reduce dose by 0.5 mg/kg (~25 mg daily) every 4 weeks until lowest effective dose is reached
BIOLOGIC DMARDs
Tumor necrosis factor (TNF) Inhibitors
- Etanercept
- Adalimumab
- Certolizumab pegol
- Golimumab
- Infliximab
Mechanism:
- Neutralizes the biological activity of TNFα by
- Binding with high affinity to the soluble and transmembrane forms of TNFα
- Inhibits binding of TNFα with its receptors
- Leads to an overall reduction in inflammation
Note: Etanercept also neutralize TNFβ (lymphotoxin α)
Dose:
Etanercept
- 50 mg SC once weekly; may be given as 25 mg SC twice weekly (individual doses should be at 3 or 4 days apart)
Adalimumab
- 40 g SC every other week; may increase dose to 40 mg SC every week
Certolizumab pegol
- 400 mg SC at 0, 2 and 4 weeks; then 200 mg every other week; may consider maintenance dose of 400 mg every 4 weeks
Golimumab
- In combination with methotrexate: 50 mg SC once per month
Infliximab
- In combination with methotrexate: 3 mg/kg IV at 0, 2, and 6 weeks, then 3 mg/kg IV every 8 weeks thereafter; may increase dose to 10 mg/kg or decrease dosing interval to as often as every 4 weeks if needed
Antirheumatic, Disease Modifying; Interleukin-1 Receptor Antagonist
- Anakinra (used only for adult Still’s disease variant of RA)
Mechanism:
- Antagonist of the interleukin-1 (IL-1) receptor
- Endogenous IL-1 is induced by inflammatory stimuli and mediates a variety of immunological responses, including degradation of cartilage (loss of proteoglycans) and stimulation of bone resorption
Dose:
Anakinra
- 100 mg SC once daily. Administer at approximately the same time each day
Renal impairment
- CrCl <30 mL/min or End Stage Renal Disease: 100 mg SC every other day
Antirheumatic, Disease-Modifying; Interleukin-6 Receptor Antagonist
- Tocilizumab
Mechanism:
- Antagonist of the interleukin-6 (IL-6) receptor
- Endogenous IL-6 is induced by inflammatory stimuli and mediates a variety of immunological responses
- Inhibition of IL-6 leads to a reduction in cytokine and acute phase reactant production
Dose:
Tocilizumab
- 4 mg/kg IV infusion over 1 hour every 4 weeks; may be increased to 8 mg/kg based on clinical response. Maximum 800 mg/infusion
Antirheumatic, Disease-Modifying; Selective T-Cell Costimulation Blocker
- Abatacept
Mechanism:
- Selective costimulation modulator
- Inhibits T-cell (T-lymphocyte) activation by binding to CD80 and CD86 on antigen presenting cells (APC), thus blocking the required CD28 interaction between APCs and T cells
Dose:
Abatacept
Note: Administered as a 30-minute intravenous infusion utilizing weight-based dosing and following the initial IV infusion, repeat IV dose (using the same weight-based dosing) at 2 weeks and 4 weeks after the initial infusion, and every 4 weeks thereafter.
- Weight
- <60 kg: 500 mg
- 60-100 kg: 750 mg
- >100 kg: 1000 mg
Biologic-Selective B cell Depleter
- Rituximab
Mechanism:
- Binds specifically to the antigen CD20 (human B-lymphocyte-restricted differentiation antigen, Bp35) on the cell surface
- Activate complement-dependent B-cell cytotoxicity; and to human Fc receptors, mediating cell killing through an antibody-dependent cellular toxicity
Dose:
Rituximab (IV infusion)
- Initial 1000 mg followed two weeks later by the second 1000 dose; subsequent courses may be administered every 24 weeks (based on clinical evaluation), if necessary may be repeated no sooner than 16 weeks following the previous course
Note: Infusions should be administered in a setting where full resuscitation facilities are available, and under the close supervision. Also administer 100 mg IV methylprednisolone 30 minutes prior to both Rituximab infusions. Acetaminophen and diphenhydramine may also be given prior to rituximab.
[/cq_vc_tab_item][/cq_vc_tabs]
Clinical Trials
Physician Resources
a) Tips for patient care
Non-pharmacologic management:
- Educate patient about RA and its management
- Emphasize correct posture and body mechanics
- Wear appropriate footwear and use assistive devices, if required
- Advise use of appropriate adaptive devices for the performance of activities of daily living when necessary
- Weight loss to decrease stress on weight-bearing joints and frequent rest periods between activities
- Take advantage of services offered by The Arthritis Society
Monitoring:
- Periodic monitoring of CBC, renal function tests, stool for occult blood (if required) is important in patients on long term NSAIDs therapy
- Periodic structured evaluation for disease activity or disease progression is recommended [pooled disease indices such as Disability Activity Score (DAS28), Simplified Disease Activity Index (SDAI) or Clinical Disease Activity Index (CDAI), as well as imaging]
- Watch for complications due to NSAIDs, such as
- GI bleeding
- Cardiovascular events
- Renal and hepatic toxicity
- Patients who are on biologic DMARDs; may carry a risk of infections, monitor and report development of any infection such as urinary tract infection or fever with sore throat etc.
- Long-term corticosteroids use increases the risk of developing osteoporosis and infections
- RA care providers should monitor disease activity as frequently as every 1 to 3 months in patients with active RA
- Patients with RA have a high cardiovascular risk related to inflammation, similar to that seen in diabetics. Control of both traditional cardiovascular risk factors and inflammation is necessary to improve cardiovascular outcomes
Medications:
- Early, aggressive treatment for RA can delay joint destruction
- DMARDs are recommended as soon as possible in active disease
- Patients on immunomodulators; may have some hair loss, diarrhea or rashes
- In prescribing medication, always evaluate risk:benefit ratio
- It advisable to have only one NSAID at a time (with the exception of low-dose cardioprotective ASA)
- Misoprostol or proton pump inhibitors are recommended in patients who are taking NSAIDs and are at increased risk for upper gastrointestinal adverse events
- Appreciate lower starting doses of drugs in elderly and debilitated patients
Social and Stress factors:
- Patient & family members should be well informed about the RA, complications, treatment and safety measures
- RA patients are prone to fatigue and muscle weakness, advise for rest when tired
- Discuss available therapeutic options and their risk and benefits with the patient
- Advise use cane of appropriate height in hand on side opposite involved hip/knee, if needed
- Educate correct posture and body mechanics
Alerts:
- Rule out septic arthritis if flare-up occurs in a single joint in established RA
- Inform patient taking NSAIDs that spontaneous GI bleeds may occur. Consider gastroprotection in high-risk patients (age >65, on ASA, prior GI ulcer/bleed)
- Discontinue NSAIDs (except ASA) in patients presenting with acute coronary syndrome
- If required, an opioid analgesic (e.g. morphine) may use as an alternative for pain control in this setting
- COX-2 inhibitors carry a reduced GI risk
- Counsel women of childbearing age regarding potential teratogenicity of DMARDs and use of effective contraception with DMARDs
Activities (physical, mental, others):
- Emphasize the importance of appropriate exercise, joint protection, strengthening of muscles supporting the joint with activity modification and safety issues to patients
- Gentle exercise can help strengthen the muscles around joints, and fatigue, e.g. swimming or gentle water aerobics
- Avoid exercising tender, injured or severely inflamed joints
- Encourage patients to participates in activities of daily living but avoid overwork and fatigue
- Keep themselves mentally active
- Encourage weight loss to decrease stress on weight-bearing joints and frequent rest periods between activities
b) Scales and Tables
- RA has been redefined as of 2010 by ACR/EULAR, based on the following criteria
- Rheumatoid arthritis and Osteoarthritis differences
- Characteristics of Synovial fluid in rheumatic disease
- Other useful tools/tables/scores used in the evaluation of RA are:
- DAS = Disease Activity Score (http://www.das-score.nl/)
- SDAI = Simplified Disease Activity Index (http://www.4s-dawn.com/DAS28/DAS28.html)
- CDAI = Clinical Disease Activity Index
- MDHAQ = Multidimensional Health Assessment Questionnaire (http://www.mdhaq.org/)
- Canadian Arthritis Referral Tool (CART) at www.rheuminfo.com
References
Core Resources:
- Aletaha D, Neogi T, Silman AJ et al.2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis & Rheumatism 2010, 69; 1580-1588
- Bykerk V P, Akhavan P, Hazelwood GS, et al. Canadian Rheumatology Association Recommendations for Pharmacological Management of Rheumatoid Arthritis with Traditional and Biologic Disease-modifying Antirheumatic Drugs. J Rheumatol 2011; 38:11; doi:10.3899/jrheum.110207
- Compendium of Pharmaceuticals and Specialties (CPS). Canadian Pharmacist Association. Toronto: Webcom Inc. 2012
- Day RA, Paul P, Williams B, et al (eds). Brunner & Suddarth’s Textbook of Canadian Medical-Surgical Nursing. 2nd ed. Philadelphia: Lippincott Williams and Wilkins; 2010
- Foster C, Mistry NF, Peddi PF, Sharma S, eds. The Washington Manual of Medical Therapeutics. 33rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2010
- Gray J, ed. Therapeutic Choices. Canadian Pharmacists Association. 6th ed. Toronto: Webcom Inc. 2011
- Katzung BG, Masters SB, Trevor AJ, eds. Basic and Clinical Pharmacology. 11th ed. New York: McGraw-Hill; 2009
- Longo D, Fauci A, Kasper D, et al (eds). Harrison’s Principles of Internal Medicine. 18th ed. New York: McGraw-Hill; 2011
- McPhee SJ, Papadakis MA, eds. Current Medical Diagnosis & Treatment. 49th ed. New York: McGraw-Hill; 2010
- Moskowitz RW, Altman RD, Hochberg MC et al. (2007). Osteoarthritis: Diagnosis and medical/surgical management.(4th ed) Philadelphia:Lippincot Williams and Wilkins
- Pagana KD, Pagana TJ eds. Mosby’s Diagnostic and Laboratory Test Reference. 9th ed. St. Louis: Elsevier-Mosby; 2009
- Skidmore-Roth L. ed. Mosby’s drug guide for nurses. 9th ed. St. Louis: Elsevier-Mosby; 2011
- Skidmore-Roth L, ed. Mosby’s nursing drug reference. 24th ed. St. Louis: Elsevier-Mosby; 2011
- Online resource/weblinks:
- The Merck Manuals
- RheumInfo
- The Arthritis Society, Canada
- Canadian Agency for Drugs and Technologies in Health
Online Pharmacological Resources:
- e-therapeutics
- Lexicomp
- RxList
- Epocrates Online
Journals/Clinical Trials:
- Aletaha D, Ward MM, Machold KP et al. Remission and active disease in rheumatoid arthritis: defining criteria for disease activity states. 2005 Sep;52(9):2625-36
- Breedveld FC, Weisman MH, Kavanaugh AF et al. A Multicenter, Randomized, Double-Blind Clinical Trial of Combination Therapy With Adalimumab Plus Methotrexate Versus Methotrexate Alone or Adalimumab Alone in Patients With Early, Aggressive Rheumatoid Arthritis Who Had Not Had Previous Methotrexate Treatment(PREMIER) Arthritis & Rheumatism Vol. 54, No. 1, January 2006, pp 26-37
- Grigor C; Capell H; Stirling A; et al. Effect of a treatment strategy of tight control for rheumatoid arthritis- the TICORA Study. The Lancet; Jul 17-Jul 23, 2004; 364, 9430
- Issues in Emerging Health Technologies: Rituximab for Rheumatoid Arthritis. Retrieved from Canadian Agency for Drugs and Technologies in Health. 2006; 89 www.cadth.ca
- Klarenbeek NB, Güler-Yüksel M, van der Kooij SM et al. The impact of four dynamic, goal-steered treatment strategies on the 5-year outcomes of rheumatoid arthritis patients in the BeSt study. Ann Rheum Dis. 2011 Jun; 70(6):1039-46
- Klareskog L; Heijde DVD; P de Jager J; Gough A; et al. Therapeutic effect of the combination of etanercept and MTX compared with each treatment alone in patient with rheumatoid arthritis(TEMPO) The Lancet; Feb 28, 2004; 363, 9410
- Lipsky PE, Desiree, Heijde VD et al. Infliximab and Methotrexate in the Treatment of Rheumatoid Arthritis (ATTRACT). N Engl J Med 2000;343: 1594-602
- Moreland, L W., O’Dell,J R., et al. Treatment of Early Aggressive RA: A Randomized, Double-Blind, 2-Year Trial Comparing Immediate Triple DMARD Versus MTX Plus Etanercept to Step-up From Initial MTX Monotherapy(TEAR). Arthritis & Rheumatism, Volume 60, October 2009 Abstract Supplement.10:1895. DOI: 10.1002/art.26968
- Rantalaiho V, Korpela M, Laasonen L, et al. Early combination disease-modifying antirheumatic drug therapy and tight disease control improve long-term radiologic outcome in patients with early rheumatoid arthritis: the 11-year results of the Finnish Rheumatoid Arthritis Combination Therapy trial(FINRACO) Arthritis Research & Therapy 2010, 12:R122
- http://arthritis-research.com/content/12/3/R122
- R F van Vollenhoven, S Ernestam, P Geborek et al. Addition of infliximab compared with addition of sulfasalazine and hydroxychloroquine to methotrexate in patients with early rheumatoid arthritis (Swefot trial): 1-year results of a randomised trial.Lancet 2009; 374: 459-66
- Schnitzer TJ, Hochberg MC, Marrero CE et al. 2011, 285-297
